Chapter 1.
Background
All of the possible products in this experiment belong to a particular class of molecules called chalcones. Chalcones (kal-cones) are biosynthesized in plants and have impressive biological activity including anti-oxidant, anti-fungal, anti-bacterial, anti-tumor, and anti-inflammatory properties.1 All chalcones share the same basic structural skeleton, which is composed of an α,β-unsaturated ketone linking two aromatic rings. Many can be isolated from plant material and can also be synthetically prepared using the aldol condensation reaction.
Reaction

1. Neves, M.P. et al. Bioorg. Med. Chem.2012, 20, 251–65
Lab Objective
This is a group experiment. You will work in a small group to design and carry out experiments in an attempt to answer one of the focus questions in the next tab. Your objective is to form and test a hypothesis in response to the focus question. The general procedure is provided and should be adapted appropriately.
Before lab discuss your focus question with your small group and answer the questions found on the group experimental design sheet, which is provided in the resources folder on CTools.
Focus Questions
Choose one of the two focus questions below (see Eğe 17.4 Section A (pp. 701–705)):
The outcome of the aldol condensation reaction is significantly influenced by the electronics of both the aldehyde and ketone.
Question 1: Which of the following aldehydes below are reactive in the aldol condensation reaction? Which are least reactive in the aldol condensation reaction?
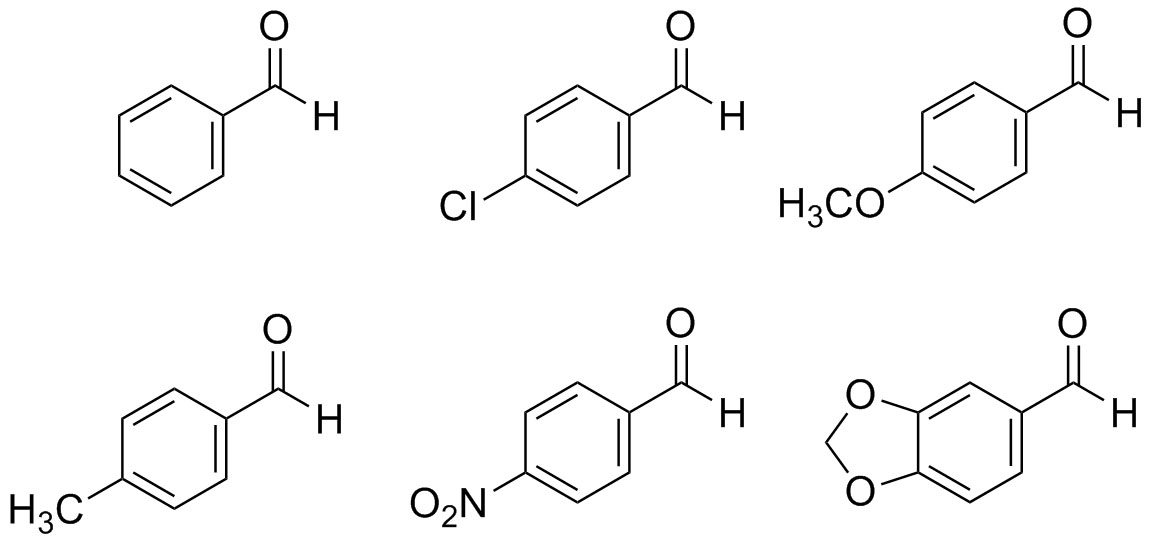
Question 2: Which of the following ketones are most reactive in the aldol condensation reaction? Which are least reactive in the aldol condensation reaction?
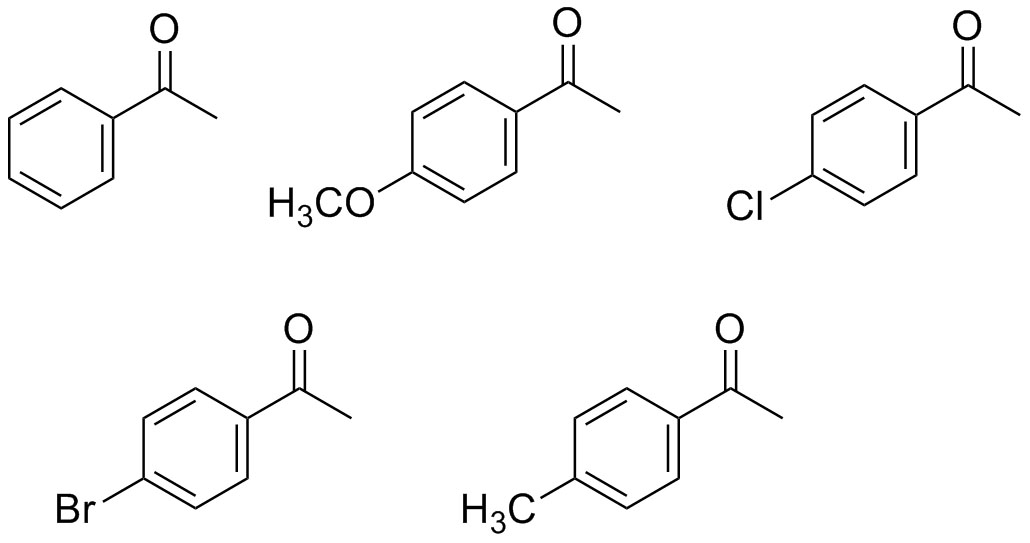
Creativity is encouraged. If you have a different idea for a focus question please discuss it with your GSI before proceeding.
Procedure
Place ~ 1 mmol (weigh accurately) of the aldehyde into a conical vial equipped with a magnetic spin vane. Add one mole equivalent amount of the ketone and 1 mL of 95% ethanol to the vial and start stirring. Add 0.10 mL of a 50% w/w aqueous sodium hydroxide solution to the vial, cap, and stir at room temperature until it solidifies (CAUTION! NaOH is a strong base). Depending on how you’ve altered the reaction conditions the reaction may take more or less time.
Most of the chalcone products will precipitate out of solution after forming. Break up the solid with a spatula and dilute with ~ 2 mL of ice water. Transfer the mixture into another ~ 3 mL of ice water in a small Erlenmeyer flask. Stir thoroughly, then vacuum filter, wash with cold water, and allow to air dry before you determine the crude yield. All aldol condensation products should be purified by recrystallization, and most can be recrystallized from 95% ethanol.
The purity of all products should be checked by TLC and m.p., and their IR spectrum recorded. Note the m.p. data for some products is not available in the literature.
Techniques
Recrystallization
Gravity & Vacuum Filtration
Thin Layer Chromatography
Infrared Spectroscopy
Table 3-1
R |
R’ |
mp (°C) |
H |
4′–OCH3 |
106 |
H |
4′–Cl |
100 |
H |
4′–Br |
104–105; 113 |
H |
4′–CH3 |
59–60; 77–78 |
4–NO2 |
H |
165 |
4–NO2 |
4′–OCH3 |
167–168 |
4–NO2 |
4′–Cl |
163–164 |
4–NO2 |
4′–Br |
166 |
4–NO2 |
4′–CH3 |
162 |
4–CH3 |
H |
96.5 |
4–CH3 |
4′–OCH3 |
? |
4–CH3 |
4′–CI |
165 |
4–CH3 |
4′–Br |
? |
4–CH3 |
4′–CH3 |
127.5 |
4–OCH3 |
H |
77 |
4–OCH3 |
4′–OCH3 |
102 |
4–OCH3 |
4′–Cl |
121–122; 128 |
4–OCH3 |
4′–Br |
142–143 |
4–OCH3 |
4′–CH3 |
94 |
4–Cl |
H |
103; 113–114 |
4–Cl |
4′–OCH3 |
130–131 |
4–Cl |
4′–Cl |
156–157 |
4–Cl |
4′–Br |
? |
4–Cl |
4′–CH3 |
? |
3,4 –O–CH2–O– |
H |
122 |
3,4 –O–CH2–O– |
4′–OCH3 |
129 |
3,4 –O–CH2–O– |
4′–Cl |
128 |
3,4 –O–CH2–O– |
4′–Br |
? |
3,4 –O–CH2–O– |
4′–CH3 |
130 |