Chapter 1. Catalase Enzyme Activity
Catalase Enzyme Activity
Catalase Enzyme Activity
by Laurel Hester and Dave Degenhart
What are enzymes? Enzymes are protein catalysts; a catalyst is a substance that speeds up the rate of a chemical reaction without being used up. Almost all chemical reactions that take place inside living cells are catalyzed by enzymes. These enzymes allow cells to control the rate of chemical reactions. In this lab you will measure reaction rates for an enzyme called catalase and investigate various factors that influence enzyme action in cells.
How do enzymes work? The basic function of an enzyme [E] is to convert a substrate [S] (or reactant) to a product [P] as described below in equation format:
[E] + [S] → [E-S] → [E] + [P]
Enzymes are highly specific; they usually only react with one substrate. This is because of the physical shape of the active site of the enzyme. The active site is the part of the enzyme where catalysis occurs. Based on the three-dimensional folding of the enzyme (recall protein tertiary structure), the active site only accommodates a substrate with a specific geometry. [E-S] represents the transition state. The transition state is a point in time during the reaction when the substrate is neither a reactant nor a product, but rather in transition between the two states. It is in the transition state that the substrate is temporarily bound to the enzyme.
To understand how enzymes can “speed up” the rate of reaction, you should recall the first two laws of thermodynamics, which tell us (1) energy cannot be created or destroyed, but it can be changed from one form to another and (2) entropy increases: energy cannot be changed from one form to another without the loss of some usable energy. Therefore, chemical reactions spontaneously occur only when the free energy of the product(s) is less than the free energy of the reactant(s). However, even spontaneous reactions may occur at extremely slow rates. For example, it is energetically favorable for diamonds to change into graphite (both are carbon crystals), but this reaction is so slow that a person does not need to worry about his or her diamond ring flaking away into grey powder. This is where enzymes come into play . . .
Let’s take as an example the chemical reaction we will be investigating today: the breakdown of hydrogen peroxide:
2 H2O2 −−−−−−−−−→ 2 H2O + O2
This reaction spontaneously occurs only when two H2O2 molecules collide with enough force to push two oxygen atoms (one from each H2O2 molecule) together in such a way that a new covalent bond between them can be formed as the old bonds are broken. Biologists and chemists describe this by saying that the two substrate molecules must have sufficient activation energy to create the transition state, or else the reaction will not proceed.
Enzymes facilitate formation of the transition state by bringing substrate molecules together, within the active site, in conformations that promote reaction progress. Furthermore, when a substrate binds to an enzyme, the active site undergoes a slight change in shape, due to electrostatic, polar, and/or nonpolar interactions with the substrate via amino acid side chains. These interactions stress critical bonds in the substrate, making it easier for bond breakage to occur. In this way, enzymes lower the activation energy necessary for the reaction to proceed. In general, all catalysts speed up reactions by lowering the activation energy (Figure 2-1).
Catalase
Enzyme Structure and Function
Catalase is one of the most efficient enzymes in your body, facilitating up to 200,000 chemical reactions per second! This enzyme catalyzes the decomposition (breakdown) of hydrogen peroxide (H2O2, the substrate) to water (H2O) and gaseous oxygen (O2). This enzyme is found in the peroxisomes of nearly all aerobic cells and protects cells from the harmful oxidizing effects of H2O2. Detoxification of H2O2 prevents fatal CO2 bubbles from forming in the blood.
The structure of catalase has been determined by X-ray crystallography; it is a tetramer containing four identical subunits (quaternary structure). Each subunit consists of 500 or so amino acids, an iron-containing heme group cofactor, and a molecule of NADH (a coenzyme). Many enzymes require the presence of cofactors and coenzymes in order to catalyze reactions. Cofactors are metal ions at various oxidation states (Fe, Mg, Zn) and coenzymes are non-protein organic molecules (NADH, NADPH, FADH2, vitamins). Both Fe and NADH are important oxidizing and reducing agents necessary for catalase enzyme function.
What Factors Influence the Rate of an Enzyme-Catalyzed Reaction?
The most obvious factors that influence the rate of enzyme-catalyzed reactions are substrate concentration and enzyme concentration. To take an extreme example, obviously no reaction will occur if no substrate is present. For a fixed enzyme concentration, the rate of reaction will increase with increasing substrate concentration until the enzyme becomes saturated (active sites of all enzyme molecules are full). Once all enzymes are saturated, the reaction proceeds as fast as that concentration of enzymes can catalyze the reaction, but further increases in substrate concentration will not cause any additional increase in reaction rate unless more enzyme molecules are added to the system.
Enzyme shape is critical to enzyme function; thus, factors that change enzyme shape affect the rate of enzyme-catalyzed reactions. For example, within a certain range, increases in temperature cause increases in reaction rate because faster moving substrates and enzymes bump into each other and bind more quickly. However, increasing the temperature beyond a certain threshold will denature the enzyme. Denaturation occurs when the hydrogen bonds and other weak interactions that determine enzyme secondary and tertiary structure are broken. Once an enzyme is denatured, the structure of the active site is destroyed, and the catalytic ability of the enzyme is lost. While some proteins can refold into their proper conformation following denaturation, others cannot.
Some enzymes can be denatured by freezing or by extreme pH conditions. Recall that pH is a measure of the hydrogen ion concentration of a solution (acidity). Free hydrogen ions in solution can interact with the R groups of the amino acids in the enzyme. Any change in the polarity or ionic strength of amino acid R groups can alter the conformation, or shape, of the enzyme. Some enzymes like pepsin (a digestive enzyme) function optimally at a low pH, but most other enzymes (including catalase) function optimally at physiological pH (~7–8).
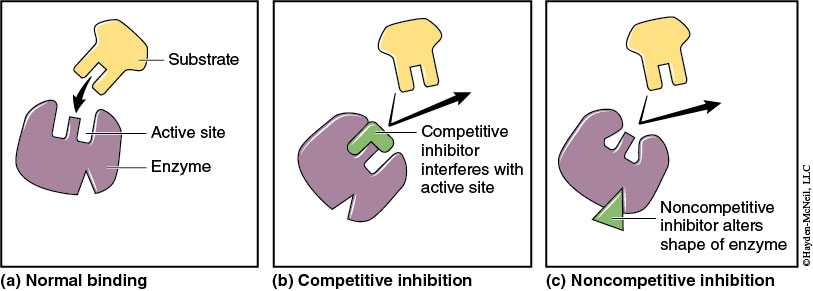
Enzyme catalysis can also be influenced by the presence of inhibitors. Generally, inhibitors prevent catalysis from occurring in two ways. Competitive inhibitors can bind directly to the active site of an enzyme due to structural similarities with the substrate. Noncompetitive inhibitors do not interact with the enzyme’s active site, but attach to the enzyme at another location, causing a change in the enzyme’s shape. Either way, enzyme function is inhibited.
Laboratory Exercise
Kinetic Analysis of Catalase Activity
This lab investigates the effects of substrate concentration and temperature on catalase enzyme activity. The activity of catalase will be measured quantitatively by observing the time required for 10 ml of oxygen (O2) to evolve as a result of the catalase-catalyzed breakdown of hydrogen peroxide (H2O2). Figure 2-2 is a picture of the system used to measure this reaction rate (reaction flask and gas collection chamber).

You will need the following materials:
- 600 ml beaker
- 10 ml graduated cylinder
- 50 ml Erlenmeyer flask with rubber stopper
- Tubing
- U-shaped glass tube
- Disposable pipettes