Chapter 1. Macromolecules
Macromolecules
Macromolecules
by Laura Jenkins, Laurel Hester, and Dave Degenhart
Throughout this lab course, we will be investigating increasingly complex levels of biological organization. At higher levels of organization, novel “emergent” properties not present in individual components arise through interactions among components. For example, each atom within the periodic table has unique properties based on the number and organization of protons, neutrons, and electrons. At the next level of organization, individual atoms combine to form water and other molecules. These molecules display unique properties not present in the individual atoms alone.
Hierarchy of Biological Organization
atoms → molecules → macromolecules → organelles → cells → tissues → organs → systems → organisms
In this laboratory exercise, you will be investigating the structure and function of macromolecules. Macromolecules are large, complex compounds that are composed of a varying number of subunits linked together by covalent bonds. Macromolecules are polymers (poly = many and mer = parts): large molecules consisting of repeating units. The individual units that make up a macromolecular polymer are called monomers.
Macromolecules exhibit great diversity, both within and among classes of macromolecules (e.g., lipids, carbohydrates, etc.). Even when subunit monomers are identical, diversity in structure and function arises from the arrangement of the individual subunits. The diversity of macromolecules is reflected in the diversity of living organisms. While the macromolecular building blocks are the same from bacteria to plants to humans (e.g., all proteins are made up of the same 20 amino acids), alternative arrangements of subunits are responsible for wildly different macromolecule functions.
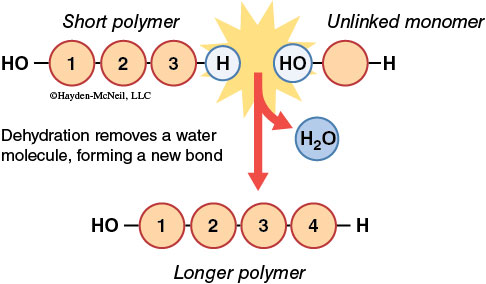
How are macromolecules synthesized? Individual monomers are linked together by a chemical reaction referred to as dehydration synthesis (also known as a condensation reaction). As the name implies, a dehydration synthesis links subunits by removing a water molecule as the covalent bond between subunits is formed. Enzymes catalyze the removal of a hydroxyl group (OH) from one subunit and a hydrogen atom (H) from the other subunit. Thus, each subunit contributes a part of the water molecule. In dehydration synthesis, one water molecule is lost for each covalent bond formed in the chain of subunits.
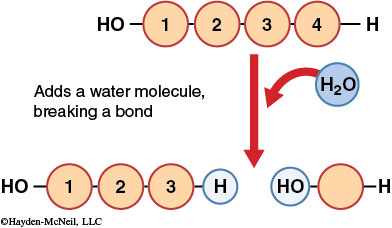
Macromolecules are broken down to individual subunits by a chemical reaction called hydrolysis (hydro = water and lysis = break). This chemical reaction breaks covalent linkages between subunits by adding a water molecule. In hydrolysis, a hydrogen atom from a water molecule is added to one subunit, and a hydroxyl group is attached to the other subunit, eliminating the covalent linkage between the subunits.
Carbohydrates, proteins, fats, and nucleic acids are the four major groups of macromolecules that make up cells.
Carbohydrates
The Sweetest of the Macromolecules
Carbohydrates consist of carbon, hydrogen, and oxygen in a 1C : 2H : 1O ratio (“carbo-” from carbon, and “-hydrate” [meaning water] from the combination of hydrogen and oxygen). Carbohydrate molecules are responsible for the storage of energy within a cellular system. They also play a role in structural support for tissues and cell-cell recognition.
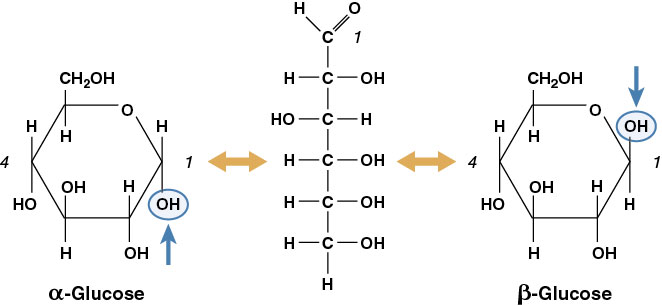
The simplest carbohydrates are the monosaccharides or simple sugars. Glucose is the most common simple sugar and the most important for living organisms. Other monosaccharides include galactose and fructose. These three have the same molecular formula, C6H12O6, but their structural formula accounts for the diversity in physical and chemical properties. When two or more compounds have the same molecular formula but differ structurally, they are termed isomers. Additionally, these substances can exist in two molecular forms, a ring structure and a straight-chain form (Figure 2-3).
Two monosaccharides can be joined together by dehydration synthesis to form a disaccharide (di = two and saccharide = sugar). The bond formed by a dehydration synthesis between two sugar units is called a glycosidic bond. Attachment of additional sugars forms a polysaccharide. Starch and cellulose are common polysaccharides consisting of many glucose subunits. Starch functions as an energy storage macromolecule in plants, whereas cellulose provides structural support for plant cells. Wood is composed of millions of compact cellulose fibers.
A polymer composed of α-glucose monomers forms starch, but a polymer of β-glucose monomers forms cellulose (Figure 2-4).
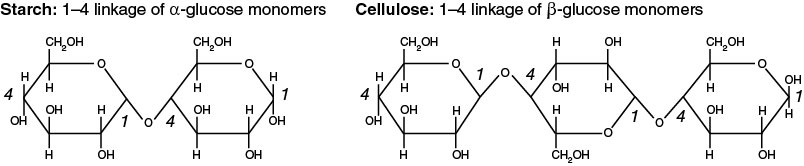
The rearrangement of the hydroxyl group of C1 accounts for the dramatic change in function of the polymer. Monomers of α-glucose can be linked in the same orientation and form a helical structure. Monomers of β-glucose are arranged in alternating orientation, causing every other glucose monomer to be “upside down,” and the whole polymer is a more linear molecule. The alternating orientation of β-glucose linkages in cellulose forms a more stable structure than that present in starch. Most organisms, including humans, cannot hydrolyze β-glycosidic bonds; this is why starch (rice, potatoes, etc.) is food but wood is not.
You will build models of simple sugar molecules and join these monosaccharides together to form polysaccharides. Refer to Figures 2-3 and 2-4 as you work!
Proteins
An Extremely Diverse Class of Macromolecules
Proteins are the most functionally diverse class of macromolecules. Enzymes, hormones, ion channels, fingernails, and contractile fibers are all proteins. All proteins in your body are made out of just 20 different amino acids. The term amino acid is derived from the structure of these protein building blocks. Amino acids have a central carbon that bonds to (1) an amino group (–NH2 ), (2) an acidic carboxyl group (–COOH), (3) a hydrogen, and (4) a variable “radical” or R-group side chain (Figure 2-5). The amino group provides the basic properties of the amino acid while the carboxyl group gives amino acids their acidic properties. All amino acids have at least one amino group and one carboxyl group. The R-group varies among amino acids and provides the identity and chemical reactivity for each individual amino acid.
The diversity of protein structure and function arises out of the endless number of sequences into which amino acids can be arranged. A group of amino acids can be joined together in a specific order to produce a given protein, just as a group of letters can be organized to create a word. A dehydration synthesis between two amino acids forms a peptide bond. This bond forms between the amino group of one amino acid and the carboxyl group of another amino acid. As with carbohydrates, when two amino acid monomers join through dehydration synthesis, one amino acid loses an H and the other loses an –OH to form H2O. Two linked amino acids form a dipeptide and the linkage of additional amino acids forms a polypeptide.
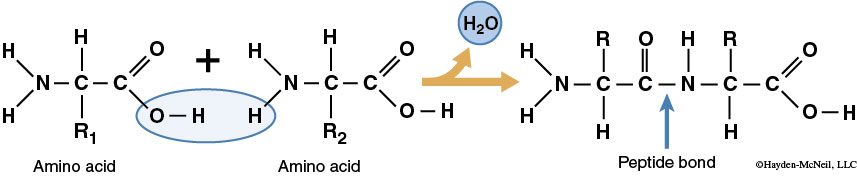
There are four levels of protein structure. The sequence of amino acids that make up the polypeptide is known as the protein’s primary structure (1°). Secondary structure (2°) is the bending and hydrogen bonding of a polypeptide backbone, which causes α-helices and β-sheets. Tertiary structure (3°) is caused by R-group interactions and defines the overall shape of the polypeptide. Quaternary structure (4°) is the interaction between several polypeptides in proteins composed of multiple polypeptides. In this lab we will focus on primary structure.
Amino acids can be categorized as essential or nonessential depending on whether they can be synthesized within the body. Essential amino acids cannot be synthesized by animals and therefore must be obtained in the diet. Humans take in proteins from various sources such as meats, fish, eggs, beans, and dairy products. When a person drinks a glass of milk, the “cow protein” is digested into individual amino acids. These pieces are then rebuilt into “human protein,” and may be used to form enzymes, build muscle tissue, etc.
You will now build molecular models in order to better visualize amino acids in three dimensions and study how peptide bonds form between two amino acids.
Lipids
Fatty Macromolecules
Lipids, also known as fats, oils, and waxes, are the third major class of macromolecules. Cells use lipids to store energy, act as insulation, form cell membranes, and regulate cell metabolism. Like carbohydrates, lipids are made up of H, C, and O. Lipids, however, have much more carbon and hydrogen than oxygen. These C–H bonds contain more chemical energy than do C–O bonds, so lipids are more energy-rich than carbohydrates. In fact, lipids supply 9 kilocalories (heat energy units) per gram as compared to only 2–4 kilocalories per gram for carbohydrates.
Lipids are much more diverse in their subunit chemistry than are other macromolecules, so our discussion will focus on the simplest of lipids: the triglyceride or fat. Triglycerides have a glycerol backbone attached to three fatty acids (Figure 2-9).
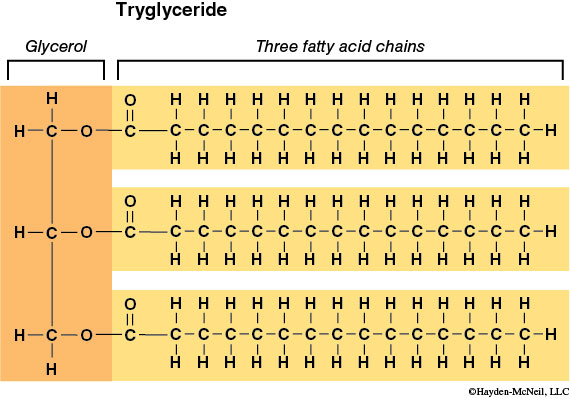
Glycerol is a 3-carbon chain with one hydroxyl (–OH) group extending from each carbon. The remaining carbon bonding sites are filled with hydrogen atoms. The second subunit, the fatty acid, consists of a hydrocarbon chain (a string of carbons surrounded by hydrogen atoms) attached to a carboxyl (–COOH) group. The fatty acid provides most of the energy-rich C–H bonds. As with other macromolecules, fatty acids are joined to the glycerol backbone by dehydration synthesis (and removed by hydrolysis). The bond formed between the glycerol –OH and the fatty acid –COOH is called an ester bond.
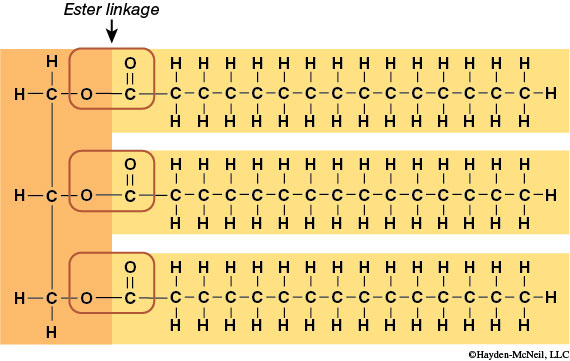
Lipids can be characterized based on the bonding within the hydrocarbon chain of the fatty acids. When all carbons are joined by single bonds, the triglyceride is described as saturated. This bonding style allows for the maximum number of hydrogen atoms to occupy all carbon bonding sites. The saturated structure is very confined and compact, tending to exist in a solid phase. When some carbons are joined to each other by double bonds, a fatty acid is said to be unsaturated. When double bonds occur, fewer bonding sites are available for hydrogen atoms to occupy. Double bonds in an unsaturated fatty acid cause kinks in the chain. Therefore, unsaturated fatty acids are not as compact and tend to occur as liquids (oils). Polyunsaturated fatty acids have more than one double bond in their hydrocarbon chain.
You will now build molecular models in order to better visualize triglycerides in 3-D and study how ester bonds form between the glycerol and the fatty acid.
Nucleic Acids
The Information Macromolecules
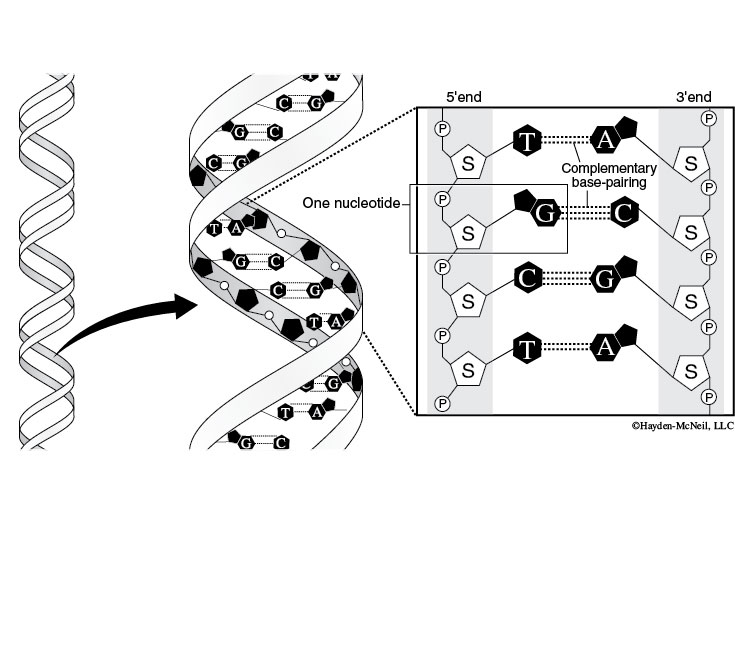
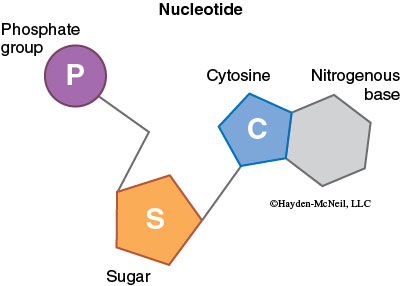
Deoxyribonucleic acid (DNA) is responsible for storing genetic information. Ribonucleic acid (RNA), made from a DNA template, directs production of all cell proteins. Nucleic acids contain C, H, O, N, and P and are formed from nucleotide monomers, which in turn are made of three subunits: a sugar, a phosphate (PO42–), and a nitrogenous base.
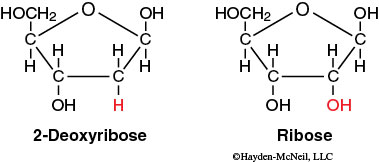
One of the key differences between RNA and DNA is that the sugar in RNA is ribose and the sugar in DNA is deoxyribose. Deoxyribose contains one less hydroxyl group than ribose; this structural difference makes DNA more stable than RNA.
Each sugar in a nucleotide is bound to one nitrogenous base and one phosphate. There are two types of nitrogenous bases: the single-ring pyrimidines (cytosine, uracil, and thymine) and the double-ring purines (adenine and guanine). DNA and RNA both contain cytosine (C), adenine (A), and guanine (G), but DNA contains thymine (T), whereas RNA contains uracil (U). This difference prevents DNA enzymes from reading RNA and vice versa.
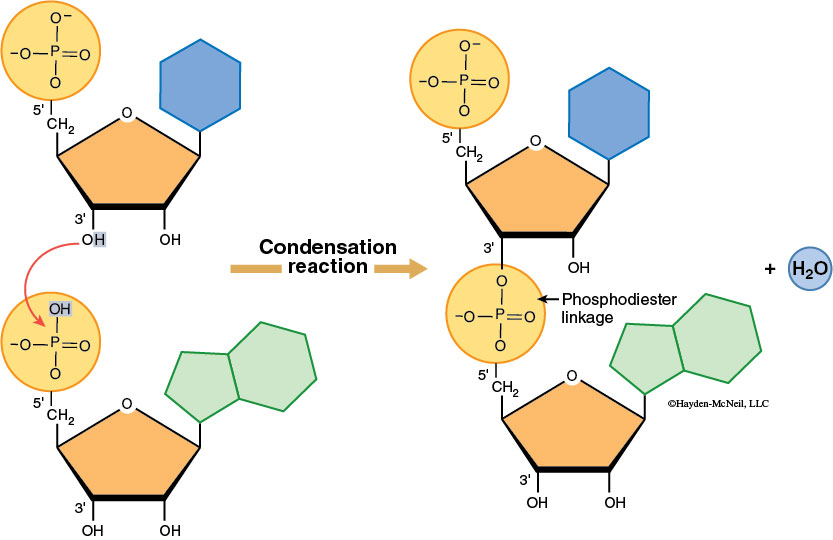
Nucleotides join to form nucleic acids through a dehydration synthesis between the phosphate group of one nucleotide and a sugar hydroxyl group. A water molecule forms as the phosphodiester bond is made. Note that the nitrogenous base is not involved in the linkage of monomer units. Nucleic acids have a sugar-phosphate backbone and the nitrogenous bases stick out from the sugars.
DNA forms a double helix due to hydrogen bonding between nitrogenous bases on adjacent anti-parallel DNA strands (this means that the strands are parallel, but they point in opposite directions. You can see this by comparing the orientation of the sugars in the figure below). Cytosine always hydrogen bonds with guanine whereas adenine bonds with thymine—this is called complementary base pairing (Figure 2-13).