Chapter 1. Diffusion and Osmosis
Diffusion and Osmosis
Diffusion and Osmosis
by Merissa Baxter and Vinal Menon
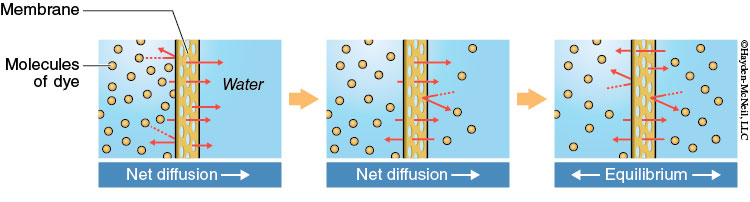
Molecules are in constant motion due to their kinetic energy. Molecules naturally move from an area where their concentration is high to an area where their concentration is low until they are evenly distributed. This process is known as diffusion. Diffusion is the spontaneous movement of molecules from an area of high concentration to an area of low concentration. Eventually these molecules become uniformly distributed. This process requires no energy.
Diffusion is a process that can also occur across a selectively permeable membrane. In nature, cells are surrounded by a selectively permeable membrane (plasma membrane) that controls what molecules are allowed to enter and exit the cell. For example, if there is a high concentration of oxygen (O2) molecules outside of the cell and a low concentration of oxygen (O2) inside of the cell, oxygen molecules will diffuse across the cell membrane and enter the cell. The O2 molecules are moving from a highly concentrated area (outside the cell) to a low concentrated area (inside the cell). They are diffusing through the cellular membrane.
Diffusion can also occur across an artificial selectively permeable membrane. Dialysis tubes (the same type of tubing used in kidney dialysis treatment) are selectively permeable. While dialysis tubing is selectively permeable to molecules based on their size, cell membranes are selectively permeable to molecules based on their size, charge, and polarity.
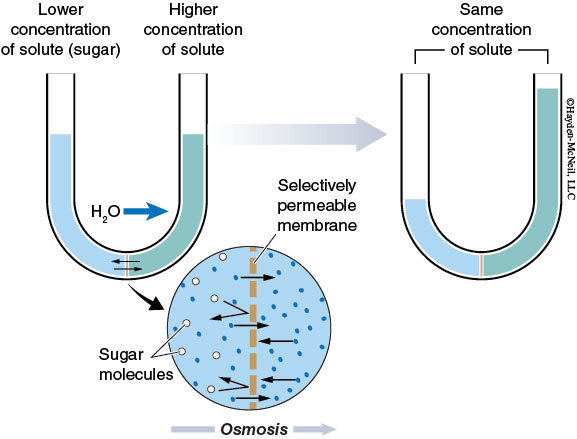
Osmosis is the movement of water molecules across a selectively permeable membrane, from a region of low solute concentration to a region of high solute concentration until the solute concentrations on both sides of the membrane are equal.
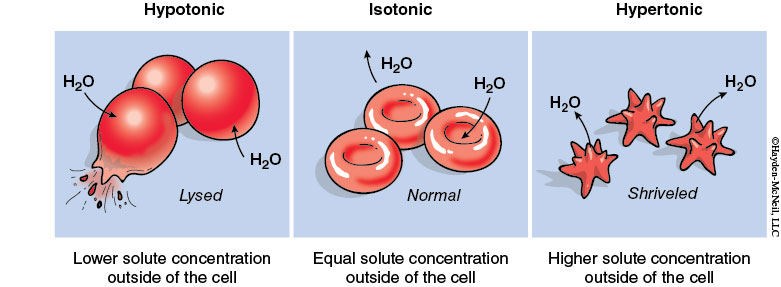
Tonicity is the ability of a surrounding solution to cause a cell to gain or lose water. Tonicity is influenced only by solutes that cannot cross the membrane. In comparison to solute concentration inside the cell, solutions outside the cell can be hypertonic, hypotonic, or isotonic.
A hypertonic solution has a higher solute concentration compared to that inside the cell (cell loses water).
A hypotonic solution has a lower solute concentration compared to that inside the cell (cell gains water).
An isotonic solution has an equal solute concentration as that inside the cell (no net water movement across the plasma membrane).
Exercise 1
Diffusion across a Selectively Permeable Membrane
In this lab you will be comparing the diffusion of two molecules across a selectively permeable membrane. These molecules are:
- Glucose—an important source of chemical energy in biological systems.
- Starch (3,000–100,000 glucose molecules linked together)—glucose is stored in this form by plants.
Your goal is to determine whether either of the molecules (glucose or starch) are small enough to diffuse through the dialysis tube. You will detect the presence of each molecule using Lugol’s and Benedict’s solutions.
- Lugol’s is used to indicate the presence of starch. It undergoes a color change from brown-yellow to dark blue or black when it reacts with starch.
- Benedict’s reagent provides a test for reducing sugars. It undergoes a color change in the presence of glucose or another reducing sugar, like maltose and lactose. To test for the presence of glucose, a small amount of Benedict’s reagent is added to the sample. Once the solution is warmed for 2–5 minutes, it should progress in changing colors from blue (with no glucose present) to green, yellow, orange, red, and then brick red or brown (with high glucose present).
Exercise 2
Osmotic Behavior of Animal and Plant Cells
All organisms must maintain an optimum internal osmotic environment. In this exercise you will compare the osmotic behavior of plant and animal cells placed in solutions with different molarity. Both animal and plant cells are bound by a selectively permeable plasma membrane. However, one of the major differences between these two cell types is the presence of a cell wall in the plant cell that confers rigidity to its structure.
Materials
Rodent blood, Elodea (a weedy aquatic plant), and the following NaCl solutions: 10%, 0.9% and 0.0%. You know that 0.9% NaCl is isotonic to most cells.
Hypothesize about the behavior of red blood cells and Elodea cells in the solutions with different concentrations of NaCl.
You will do the experiments by making up a slide with either one drop of diluted rat blood or one Elodea leaf and adding one drop of the appropriate salt concentration. Note whether you accept or reject your initial hypothesis. Observe all combinations of cells and salt concentrations and fill out the table in the worksheet.