Chapter 4. Osmosis and Diffusion: Testing a Hypothesis with a Model System
Objectives
By the end of the period, students will be able to:
- understand the importance of osmosis and diffusion in living systems.
- complete an experiment using dialysis tubing to model diffusion.
- understand and explain the process of hypothesis testing.
Osmosis and Diffusion
In this lab we will build on everything that you have already learned in lab. We will use microscopes, make a wet mount, use the process of hypothesis testing, and model an important biological/chemical process!
Have you ever smelled a skunk without even seeing where it is? If so, you have experienced the process of diffusion. The potent chemicals that make the smell are produced in specialized glands and are released when the skunk is threatened. Or, have you ever had a sprained ankle and been told to soak your injury in Epsom salts? In so doing, you are drawing fluids out of the injured joint and often easing the pain. If so, you have experienced osmosis.
Diffusion is the movement of a substance from a region of high concentration to a region oflower concentration. Both gases and liquids diffuse in essentially the same way, although diffusion happens much more quickly for gases. Diffusion is critically important in the functioning of many biological systems. Oxygen diffuses into cells, where it is used in respiration. Carbon dioxide diffuses out of cells into the bloodstream and is then taken to the lungs, where it is exhaled. Your TA will provide other examples.
If there are materials dissolved in a solution, those materials are called the solutes. Let’s compare two solutions. If the first solution is deionized water, there are no solutes in it and it is 100% water. If a second solution has glucose dissolved in it, the solute is the glucose and the concentration of water is less than 100%. If there were a permeable membrane between these two solutions, the glucose would move by diffusion from solution 2 to solution 1. However, since the concentration of water is greater in solution 1, there will be diffusion of water from solution 1 to solution 2. Both the glucose and water will move if the membrane is permeable. In reality, most membranes are selectively permeable, allowing substances only of a certain size to move.
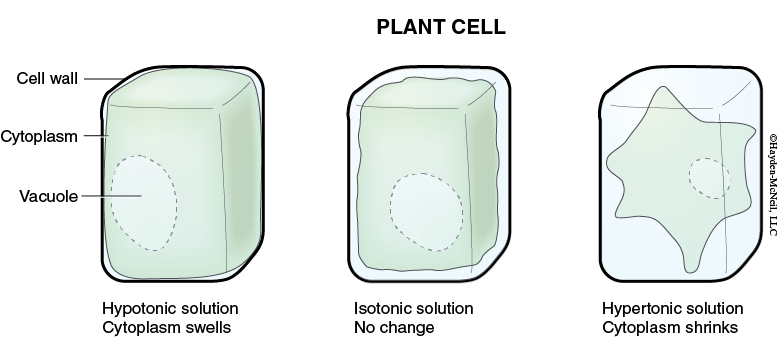
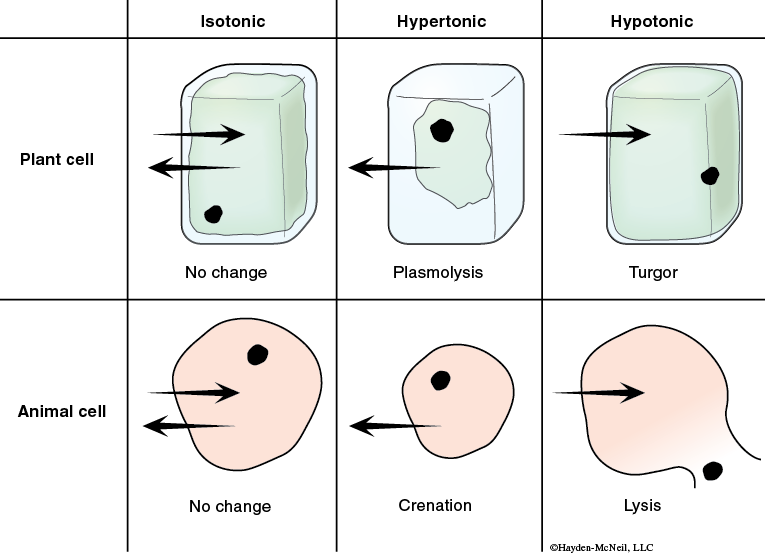
When we compare two solutions relative to each other, we often use the terms hypotonic, hypertonic, or isotonic. If two solutions have the same concentration of solutes, they are isotonic relative to each other and if the solutions are next to each other, they won’t change (Figures 4.1 and 4.2).
If one solution has more solutes (and thus a lower concentration of water), it is hypertonic relative to the other solution. And, if these two solutions are separated by a selectively permeable membrane, there will be a net movement of water into the hypertonic solution.
If a solution has fewer solutes than another solution, it is said to be hypotonic relative to the other solution. And, if these two solutions are separated by a selectively permeable membrane, water will leave the hypotonic solution and move into the hypertonic solution.
Remember when using the terms isotonic, hypertonic, and hypotonic, they are always comparative. Thus if you say one solution is hypertonic, it has to be in comparison to another solution.
Osmosis is the diffusion of water across a selectively permeable membrane. The plasma membrane of cells is an example of a selectively permeable membrane. Water will move into or out of cells depending on the concentration of water that surrounds the cell. The cytoplasm of a cell is mostly water, but there are lots of other molecules in the cytoplasm. If a cell is placed in fresh, pure water, the concentration of water inside the cell is less than outside and water will diffuse into the cell. (Note: The cell is hypertonic relative to the fresh, pure water. The water is hypotonic relative to the cell.)
Laboratory Procedure
1. CAREFULLY EXAMINE AND UNDERSTAND THESE EXAMPLES.
Examples. If you place a drop of saltwater next to the leaf of an Elodea (an aquatic plant), water will move out of the plasma membrane and into the saltwater. That is, water diffused out of the plasma membrane, causing it to shrink. Why is this so visible in plant cells? What do plant cells have that animal cells do not?
Several slides will be set up in advance. Below is a description of each slide. For your Hand-in, you will draw these cells and describe what is occurring.
- A wet mount of Elodea in fresh water. What do you expect to happen? Describe the Elodea relative to the water. Make a sketch of this on your Hand-in.
- A wet mount of Elodea in a 10% salt solution. What do you expect to happen? Describe the Elodea relative to the salt water. Make a sketch of this on your Hand-in.
- A wet mount of sheep’s blood in a 0.9% salt solution. What do you expect to happen? Describe the sheep’s blood relative to the 0.9% salt solution. Make a sketch of this on your Hand-in.
- A wet mount of sheep’s blood in a 10% salt solution. What do you expect to happen? Describe the sheep’s blood relative to the 10% salt solution. Make a sketch of this on your Hand-in.
- There will be several potato slices in lab, each soaked in a different solution. See how each feels and explain the differences in your Hand-in.
2. WHAT AFFECTS THE RATE OF DIFFUSION?
We will use dialysis tubing as a means of measuring diffusion. Your TA will show you how diffusion can be measured. Dialysis tubing is selectively permeable (water can pass through it, but larger molecules such as starch cannot pass through the membrane) and so it is a goodmodel of the plasma membranes of cells. Why are models important in science?
Many different things could possibly affect the rate of diffusion. For example, what if we used a higher concentration of sucrose in one of the solutions? Would that affect the rate of diffusion? Could temperature be important? In this next part of the lab we will do an experiment to investigate this.
Experimental Design
Question: What will happen to the rate of diffusion if the sucrose concentration is higher than 10%?
Hypothesis. State what you think will happen. Make a prediction and put it in the form of a statement.
Question
pSs1ox3kKTTKDgtmq0vtctOT6k/l+SrMQPemBOUars4GCjDCtxo/qrdNCi5zGNut8GPoerWsVmomq95b1YtEdhZIZdByVTatmr945VTB8vg=Question
f29XUNBhIfAp6+HA0dA8v7BhtE77iWJYRpKZmDMrXYEeosXGVNneFKwBdPfGX2fQkGIWBpO+3OZbXqEySl48Mk8jut4HGI7hj3JqaqLfUJJzIk8HS4zM5g==Each lab group will have the following materials: Dialysis bags and 3 cups for water. The TA will precut the bags and will prepare the solutions for the experiment (0% sucrose, 10% sucrose, and 20% sucrose).
Basic Procedure
Your TA will demonstrate by making one bag. Three 15 cm long dialysis bags will first be soaked in water. Then for each, you will twist one end shut and tie it with a knot. It is critical that the knot be tight, and that no fluid can leak out. Ten ml of each of three solutions will be pipetted into each bag. Air is gently squeezed from the top of the bag. Twist, fold, and tie the free end of the tubing as you did before. The bag should be leak proof! Gently blot the excess water off each of the bags and weigh them separately on the balance provided. Record the mass of each at time “0.” The bags are then placed into the appropriate cups of water. The mass is recorded every 5 minutes in Table 4.1 on your Hand-in.
For this experiment, we will use 10% sucrose and 20% sucrose, as well as water as the control. In this example, the independent variable is the concentration of sucrose in the bag. The dependent variable, what we will measure, is the rate of diffusion. Since many lab groups will do the same experiment, we will have several replicates.
A second alternative experiment can be performed using temperature as a variable. If the class chooses this option, you will complete Table 4.2.
WHEN YOU HAVE COMPLETED THIS EXPERIMENT
Each lab must be clean and ready for the next group of students. Part of your grade will be based on how clean the lab is left for the next group!
CHECKLIST FOR THE END OF LAB
❑ Clean up your lab table. Make sure microscope slides and coverslips are clean.
❑ Wipe up your table and make sure there are materials ready for the next group. Follow the instruction of your TA in getting the materials ready for the next group.
❑ Clean up around the sinks.