Chapter 5. Enzymes: Digestion as a Model System
Objectives
By the end of the period, students should be able to:
- understand some of the dynamics of how enzymes work.
- understand the importance of enzymes in digestion.
- plan and execute an experiment on the effects of an environmental variable on the action of amylase on starch.
Thanks to Dr. Denis Goulet of the University of Mississippi for helping to develop this exercise.
Background
Life would not be possible without enzymes. Reactions could happen, but at such a slow rate, that life could not happen. Most chemical reactions in living organisms, including the process of digestion, require enzymes. Most enzymes are proteins and these are biological catalysts: they greatly increase the rate of a chemical reaction but are not themselves changed during the process.
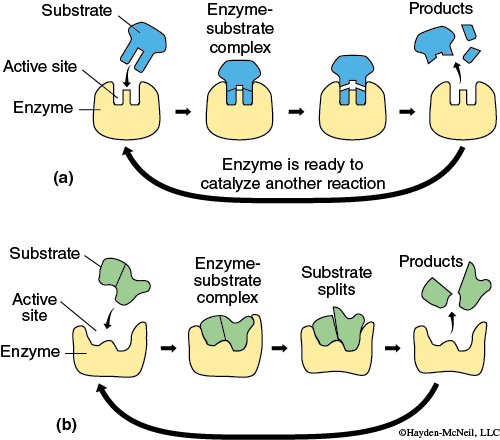
Enzymes speed up a wide variety of reactions, including hydrolysis, decomposition, and oxidation. The substrate of a reaction is the chemical constituents that are catalysed by an enzyme. In general, an enzyme will bind to a substrate to form an enzyme-substrate complex and in the reaction that follows, the substrate is changed to products. Remember that the enzyme is not used up in the process.
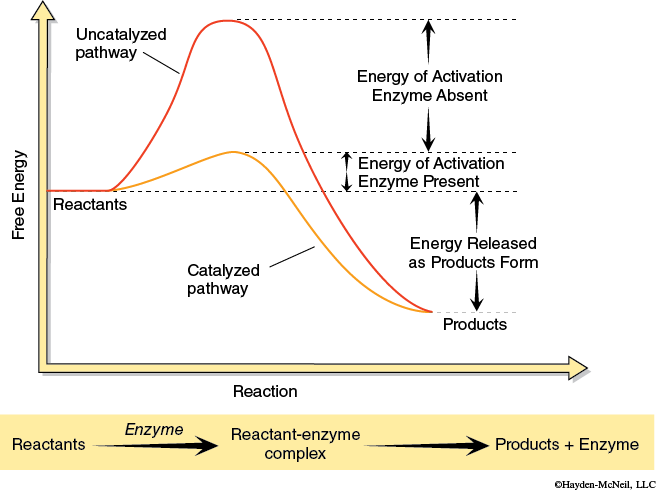
Enzymes are remarkably specific and will bind only with one or a few substrates. Figure 5.1 shows the process of an enzyme binding with a substrate and products being formed. Figure 5.2 shows how an enzyme works by lowering the energy of activation needed for a reaction. The rate of reaction depends on a variety of factors, but often there is a period when the rate increases in a linear fashion, then levels off, and then the rate decreases as the substrate is used up.
Many enzyme names end in the suffix “ase” and in general, when you see a word that ends in “ase,” it is an enzyme. We will model the reaction rate using an enzyme called “toothpickase.”
Procedure Part 1. "Toothpickase"
The first part of our exercise today will be to demonstrate the rate of reactions. We will do this by breaking toothpicks. Our goal will be to break as many toothpicks as possible in a 2-minute period. The toothpicks are the substrate, the enzyme is your hands, and the product is the broken toothpick. Note that the enzyme (your hand) is much larger than the substrate (the toothpick) and note also that if this is done correctly, the enzyme (your hands) are not changed by this process!
Here are the rules:
You can only break one toothpick at a time, by holding it with both hands and breaking it.
Each toothpick must be broken completely in half. You must keep your eyes closed.
You must start and stop when your TA says “start” and “stop.”
As you do this, please note: The toothpicks only break if you find just the right spot to break them.
You will work in teams of two. One person (called the “counter”) will spread out 50 toothpicks on the lab table; the second person must close their eyes and be the enzyme.
When the TA says “start,” the enzyme will break as many toothpicks as possible until the TA says “stop” after 10 seconds. The counter will count and record the number of broken toothpicks but will not remove them. The TA will again say “start” and will say “stop” after 10 more seconds (for a total of 20). The counter will again count and record the broken toothpicks. The TA will time you for 10 more seconds and that will continue in 10 second increments.
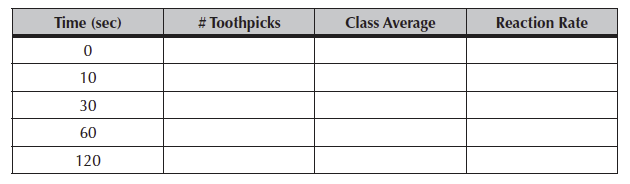
The TA will then add the numbers together from the lab teams and everyone will calculate the reaction rate for each of the time periods.
The reaction rate is (M2 – M1)/(T2 – T1)
Where “M” is the number of tooth picks metabolized (broken) and “T” = time.
The data can be recorded in Table 5.1 below and then copied to your Hand-In.
Graph the time on the x-axis and the rate of reaction on the y-axis and answer the questions about this exercise.
What if the toothpicks were spread in a bigger area? What if your hands were very cold? What if you had to wear gloves? What happens when most of the toothpicks were broken? Did your rate slow down or speed up?
Table 5.1. Reaction time for breaking toothpicks.
“Toothpickase” used with permission. Thanks to Jim Billingsley and Lonnie Miller.
Enzymes Important in Digestion
Many enzyme-mediated chemical reactions occur inside of cells, such as cellular respiration, fermentation, or photosynthesis; the chemical reactions of digestion may occur inside or outside of cells depending on what kind of organism you are. In most animals, digestive enzymes are secreted into a special extracellular (outside of cells) cavity called the gut, where digestion actually takes place. The small molecules released by digestion can then be absorbed by the circulatory system and distributed to cells throughout the body. The smaller molecules diffuse from the gut (mostly from the small intestine) into the circulatory system where they are then taken to the liver and then to cells all over the body. From the blood, the nutrients diffuse into cells. Once inside a cell, these small molecules can be modified and reassembled to form molecules required by the consumer.
In this part of the lab, we will learn methods to measure digestion of a complex carbohydrate (starch). Also, your research group will design and carry out an experiment to investigate the effect of an environmental factor on the rate of digestive enzyme activity.
Enzymes are important in digestion. We will discuss three different enzymes today but will only do a demonstration and experiment with one. You should know the importance of proteins, lipids, and carbohydrates for human nutrition.
DIGESTION OF PROTEIN BY PEPSIN
Proteins are important as a structural element in bones, cartilage, hair, feathers, nails, and cell membranes. They are also important as enzymes, hormones, antibodies, and in oxygen transport in red blood cells. Proteins are formed by the linkage of amino acids into polypeptides. A protein may be composed of one or several polypeptides wrapped together into a precise structure.
Chemical digestion of proteins begins in the stomach. The acidity of the stomach fluids is very high (low pH). The primary enzyme for protein digestion in the stomach, pepsin, works best at low pH. Further protein digestion is carried out in the small intestine by trypsin, which is secreted by the pancreas. Any enzyme that digests proteins is a protease. As proteins are digested, the polypeptide chains unravel and break up into small chains of amino acids called peptides.
DIGESTION OF LIPIDS BY PANCREATIN
Lipids include fats and oils. They are a fundamental component of cell membranes and may be used for energy storage or insulation. A characteristic feature of lipids is that they do not dissolve in water but may dissolve in nonpolar compounds. Lipid digestion occurs in the small intestine. It must begin by making the molecules more compatible with water so that the digestive enzymes have access to them. This is accomplished by breaking up the lipid into small droplets, which can be distributed in the water of the small intestine. The process of breaking up a molecule into small droplets immersed in water is called emulsification; the mixture that results is called an emulsion. In the small intestine, a substance called bile emulsifies lipids. Bile is produced in the liver, stored in the gall bladder, and pumped into the small intestine when lipids are present.
Once lipids are emulsified, they may be digested into their subunits (glycerol and fatty acids) by digestive enzymes called lipases. Lipases are produced in the pancreas and secreted into the small intestine. The subunits produced from lipid digestion can be absorbed by the circulatory system and distributed to cells.
DIGESTION OF STARCH BY AMYLASE
Carbohydrates include simple sugars such as glucose and sucrose and polysaccharides such as starch and cellulose. They are important as structural compounds and as a source of energy that can be used to make ATP. Starch is a complex polysaccharide made in plant cells for the storage of energy. Foods such as potatoes and pumpkins are very rich in starch, so these foods are good sources of energy for plant-eating consumers like you. Cellulose is one of the most common of all carbohydrates. It is found in the rigid cell wall surrounding plant cells. Our digestive systems cannot break down cellulose. Because it is indigestible, cellulose is the largest component of dietary fiber. It adds bulk to the intestinal contents and thus helps the remains of meals pass through the digestive system more easily. Seeds often have a large amount of starch as a means of storing energy for the developing plant. Seeds will also produce the enzyme amylase to break the starch down into simple sugars that the plant can then use.
Digestion of carbohydrates (including starch) converts polysaccharides (long chains of simple sugars) and disaccharides (two sugars linked together) into monosaccharides (single sugar units) that can be absorbed into body cells. Carbohydrate digestion begins in the mouth and is completed in the small intestine. Carbohydrates are not digested in the stomach. Can you imagine why?
In this exercise, we will demonstrate the effect of amylase on the rate of digestion of starch. Amylase is an enzyme produced in the mouth and in the small intestine where it clips polysaccharides into disaccharides and monosaccharides. Iodine reagent, I2KI (iodine-potassium iodide), which turns dark blue in the presence of starch, will be used to determine whether starch digestion has occurred. If no starch is present the solution remains yellow. If the starch has been partially digested, a red-brown color will result. Your TA will demonstrate the presence of starch in a potato to show you what the iodine looks like in the presence of starch.
Procedure Part 2
- DEMONSTRATION OF THE USE OF IODINE AS AN INDICATOR SOLUTION AND DEMONSTRATION OF STARCH DIGESTION
This first portion will be demonstrated by your TA.
Start with two test tubes. The tubes should be labeled 1 and 2.
- Measure 3 ml of 3% starch into each tube.
- Add one drop of iodine to each tube. The starch should turn black.
- Add three drops of water to tube 1. This is your control tube.
- Add three drops of amylase to tube 2. This is the experimental tube.
- Put the cap on, shake each tube, and immediately start timing.
At one-minute intervals, shake the tube and record the color.
In complete sentences, describe what happens to each tube.
Repeat this procedure. What was different in the second run?
- EXPERIMENT DESIGN AND PROCESS
How does temperature affect the rate of a reaction? The class as a whole will do an experiment to investigate this question.
What would you guess would be an optimal temperature for most of the enzymes in our bodies?
Each team will have access to room temperature water, an ice chest, and a hot water bath.
Working with your TA, you will identify your hypothesis, your prediction, your control, and your independent and dependent variables.
Each team will do the following and your TA will collect everyone’s data.
- Measure the temperature of the water or ice that your reaction tubes will be in.
- Measure 3 ml of 3% starch into each tube.
- Add enough iodine (one drop at a time) until the solution turns black (or a 5 on the color chart). If the solution never turns black, dump it out and start again with a different dropper bottle of iodine.
- Add amylase as instructed by your TA, or water if you have the control.
- Put the cap on, shake each tube, and immediately start timing. Be sure to keep the tubes in the water bath or ice.
At one-minute intervals, shake the tube and record the color.
How long does it take for the tubes to turn from black to brown to yellow? Note each color change and record the time.
Rinse out your tubes and pipettes when you are finished.
NOTES TO INSTRUCTORS AND MATERIALS FOR LAB
Each lab must have the following:
- Hot water bath
- One ice chest for each table
Each table has the following:
2 droppers with starch
- 2 iodine
- 2 water
- 2 test tube racks, each with 2 plastic test tubes, each with a plastic 1 ml pipette
- Sheet showing color change of starch digested by amylase
Amylase will be on the side bench.
NOTES TO TAs
Before lab, you should run the reaction to see how long it takes. There are many variables that affect it. You can then adjust the number of drops to make the reaction run in a reasonable length of time for the students (1–5 minutes).
Next, have your students run the reaction to see the effect of amylase on the breakdown of starch. They need to understand the role of the substrate, the enzyme, and the indicator solution.
Finally, have your students run one or more experiments to see the effects of temperature on the rate of the reaction. Use this as a time to reinforce good planning of an experiment and of reporting the results. Students should make graphs of color changes over time.
At the end of the lab, each table must clean up their materials and leave it ready for the next group.