Chapter 1. Experiment 11
Qualitative Inorganic Analysis: Groups 1 and 2 Known
Purpose of the Experiment
To separate and identify representative cations from Qualitative Analysis Groups 1 and 2.
Background Required
You should be familiar with techniques found in the Qualitative Inorganic Analysis Preview.
Background Information
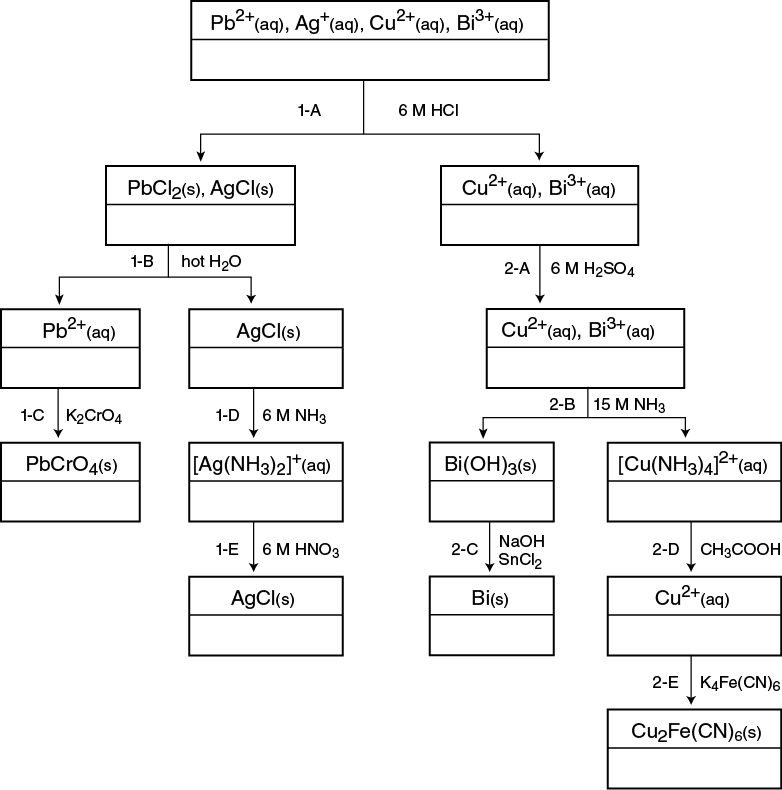
Group 1 cations are grouped according to the chloride solubility rule, that all chloride salts are soluble except AgCl, PbCl2, and Hg2Cl2. Hence the three cations in Group 1 are Ag+, Pb2+, and Hg22+. However, in this analysis you will only analyze for Pb2+ and Ag+. Group 1 cations are separated from all other cations in a mixture by adding Cl– ions to precipitate AgCl and PbCl2. These solids form and settle to the bottom of the container.
Group 2 cations precipitate as insoluble sulfide salts from acidic solutions. The seven cations in Group 2 are: Hg2+, Cu2+, Cd2+, Bi3+, As5+, Sb3+, Sn4+. However, you will analyze for Cu2+ and Bi3+. Traditionally, generating small amounts of H2S, a very toxic and foul-smelling gas, precipitates Group 2 cations. To avoid using H2S, this analysis has been changed to eliminate steps involving sulfide salts.
Groups 1 and 2 Procedure Background
1–A: Separation of Group 1 (PbCl2 and AgCl)
Chloride ions (from HCl) precipitate Pb2+ and Ag+ as their insoluble chloride salts, PbCl2 and AgCl, thus separating them from Cu2+ and Bi3+. Step 1–A2 checks for complete precipitation. After centrifuging the mixture, the supernate (Cu2+ and Bi3+) is decanted off the solid (PbCl2 and AgCl) and set aside for further analysis in 2–A.
Ag+(aq) + Cl–(aq) → AgCl(s)
Pb2+(aq) + 2 Cl–(aq) → PbCl2(s)
1–B: Separation of Pb2+
PbCl2 is three times as soluble in hot water as in cold water, but the solubility of AgCl is not affected by changes in temperature. Thus PbCl2(s) dissolves in hot water producing Pb2+ ions in solution, separating it from the unaffected AgCl(s). Centrifuging and decanting must be done while the mixture is hot.
PbCl2(s) → Pb2+(aq) + 2 Cl–(aq)
1–C: Confirmation of Pb2+
The addition of K2CrO4 in a weakly acidic solution precipitates Pb2+ ions as PbCrO4. The formation of a bright yellow precipitate confirms the presence of Pb2+ in the solution.
Pb2+(aq) + CrO42(aq) → PbCrO4(s)
1–D and 1–E: Confirmation of Ag+
The presence of Ag+ ions is confirmed by the reprecipitation of AgCl(s) in two steps. In 1–D, NH3(aq) is added to dissolve AgCl(s), forming Ag+ and Cl– ions. The Ag+ ion further reacts with the NH3(aq) to form the complex ion, [Ag(NH3)2]+(aq). (Any remaining solid, undissolved PbCl2, is discarded). In 1–E, HNO3 is added to break up the complex ion and allows the Cl– ions to precipitate the Ag+ as AgCl(s). The appearance of a white precipitate confirms the presence of Ag+ in solution.
AgCl(s) + 2 NH3(aq) → [Ag(NH3)2]+(aq) + Cl–(aq)
[Ag(NH3)2]+(aq) + Cl–(aq) + 2 H+(aq) → AgCl(s) + 2 NH4+(aq)
2–A: Removal of Trace Amounts of Pb2+
Sulfuric acid is added in 2–A to precipitate any trace amounts of Pb2+ as PbSO4(s). The sample is centrifuged and the supernate kept for step 2–B, and the solid, PbSO4 is discarded.
2–B: Separation of Bi(OH)3
When concentrated NH3 is added in 2–B, two separate reactions occur. The Bi3+ ion precipitates from solution as Bi(OH)3(s) while the Cu2+ ion forms [Cu(NH3)4]2+(aq). Centrifuging and decanting separates the white solid Bi(OH)3 from the dark royal blue solution of [Cu(NH3)4]2+. The presence of a white precipitate indicates, but does not confirm, the presence of Bi3+. The presence of a deep royal blue solution indicates, but does not confirm, the presence of Cu2+.
Bi3+(aq) + 3 OH–(aq) → Bi(OH)3(s)
Cu2+(aq) + 4 NH3(aq) → [Cu(NH3)4]2+(aq)
2–C: Confirmation of Bi3+
In basic solutions, Sn2+ reduces Bi3+ to its elemental state of Bi(s). The immediate formation of a black solid, Bi(s), confirms the presence of Bi3+.
2 Bi3+(aq) + 3 Sn2+(aq) → 2 Bi(s) + 3 Sn4+(aq)
2–D: Dissociation of [Cu(NH3)4]2+
In step 2–D, [Cu(NH3)4]2+(aq) is acidified with CH3COOH to dissociate the complex ion and form Cu2+ ions. The sky blue color solution is characteristic of Cu2+ ions.
[Cu(NH3)4]2+(aq) + 4 H+(aq) → Cu2+(aq) + 4 NH4+(aq)
2–E: Confirmation of Cu2+
The addition of K4Fe(CN)6 to the Cu2+ solution precipitates the maroon Cu2Fe(CN)6 solid. The formation of the maroon copper(II) ferrocyanide salt confirms the presence of Cu2+.
2 Cu2+(aq) + [Fe(CN)6]4–(aq) → Cu2Fe(CN)6(s)
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Groups 1 and 2 Known Solution
Dispose of all solutions and solids in their appropriate waste containers.
DO NOT POUR ANYTHING DOWN THE DRAIN!
Read the Qualitative Inorganic Analysis Preview section in the online materials. It provides background vocabulary, background techniques, and a Sample Data Sheet. It details how to record your observations and match the corresponding formulas of the solids and/or ions in the data sheet. The chemical formulas are found in the Background Information section for this experiment online and they are also provided in the flowchart on the next page.
Record your observations and corresponding chemical formulas of the solids and/or ions for each step of the procedure. Record appropriate inferences as you proceed in your notebook for this experiment.
Always used distilled water whenever water is used.
Groups 1 and 2 Flowchart
Part I
Start a Hot Water Bath with Distilled Water
1–A: Separation of Group 1 Cations
1–A1 Add 10 drops of the known cation solution to a small test tube.
Add 4 drops of 6 M HCl to the test tube.
Stir thoroughly and centrifuge.
1–A2 Without disturbing the solid, add 2 drops of 6 M HCl to the solution. Watch for any cloudiness forming in the solution. (This indicates incomplete precipitation.) If no change is observed, proceed to the next step. If some precipitate forms, add 2 additional drops of 6 M HCl, stir, and centrifuge.
1–A3 Decant the supernate into a clean test tube.
Label this test tube 2–A and set aside for later use.
1–A4 Wash the precipitate from the previous step with 10 drops of distilled water.
Centrifuge and discard the decantate in the waste container.
Keep the solid for the next step.
1–B: Separation of Pb2+
1–B1 Add 14 drops of hot distilled water (from your water bath) to the precipitate from 1–A4.
Place the test tube in the hot water bath and stir often, while heating for 1 minute.
1–B2 Centrifuge the mixture while it is hot.
Decant the supernate into a clean test tube and keep for 1–C1.
Keep the solid for 1–D1.
1–C: Confirmation of Pb2+
1–C1 Add 2 drops of 6 M CH3COOH to the decantate from the previous step.
Add 5 drops of 0.2 M K2CrO4 to the solution and stir.
1–C2 Formation of a yellow precipitate (not just a yellow solution) confirms the presence of Pb2+.
Show your results to your instructor for their initials.
Discard the contents of the test tube in the waste container.
1–D: Detection of Ag+
1–D1 Add 10 drops of 6 M NH3 to the precipitate from step 1–B2.
Stir well and centrifuge.
1–D2 Decant the supernate into a clean test tube and save for 1–E1.
Discard any solid that remains.
1–E: Confirmation of Ag+
1–E1 Add 15 drops of 6 M HNO3 to the solution from 1–D2.
Stir well and check that the solution is acidic to litmus.
If the solution is not acidic, add additional drops of 6 M HNO3 until the solution is acidic.
Stir and centrifuge.
1–E2 Formation of a white precipitate confirms the presence of Ag+.
Show your results to your instructor for their initials.
Discard the contents of the test tube in the waste container.
2–A: Removal of Pb2+
2–A1 Add 3 drops of 6 M H2SO4 to the solution from step 1–A3.
Stir well and wait for 1 minute. Centrifuge the mixture.
2–A2 Decant the supernate into a clean test tube and save for 2–B1.
Discard any solid that remains.
2–B: Separation of Bi(OH)3
2–B1 In the hood, add 15 M NH3 dropwise to the solution from 2–A2.
Continue adding the 15 M NH3 until the solution is basic to litmus. Add 3 additional drops of 15 M NH3 in excess to the solution.
2–B2 Stir for 2 minutes. Centrifuge and decant the supernate into a clean test tube, and save for 2–D.
The presence of Cu2+ is indicated, but not confirmed, by the appearance of a deep royal blue color in the decantate.
2–B3 Wash the precipitate from the previous step with 20 drops of distilled water. Stir well, centrifuge, and discard the supernate in the waste container.
2–B4 Repeat step 2–B3 to wash the precipitate again.
A white precipitate indicates, but does not confirm, the presence of Bi3+. If trace amounts of a blue solution remain, the precipitate may appear light blue.
2–C: Confirmation of Bi3+
2–C1 Add 6 drops of 6 M NaOH and 4 drops of 0.2 M SnCl2 to the precipitate from 2–B4. Stir thoroughly and centrifuge.
2–C2 Formation of a black precipitate confirms the presence of Bi3+.
Show your results to your instructor for their initials.
Discard the contents of the test tube in the waste container.
2–D: Dissociation of [Cu(NH3)4]2+
2–D1 Add 6 M CH3COOH dropwise to the solution from step 2–B2, until the solution is acidic to litmus.
2–E: Confirmation of Cu2+
2–E1 Add 2 drops of 0.2 M K4Fe(CN)6 to the solution from 2–D1.
Stir well and centrifuge.
2–E2 Formation of a maroon precipitate confirms the presence of Cu2+. Show your results to your instructor for their initials.
Discard the contents of the test tube in the waste container.
Study Questions
1. a. HCl is added to a Pb2+ solution and a solid forms. What is the formula of the solid?
b. HCl is added to a Ag+ solution and a solid forms. What is the formula of the solid?
2. a. AgCl(s) is in one test tube and PbCl2(s) is in another. Hot water is added to both test tubes. Describe what will occur in each of the test tubes. If a change or reaction occurs, write the new chemical formula.
b. Cu2+(aq) is in one test tube and Bi3+(aq) is in another test tube. Aqueous ammonia is added to both test tubes. Describe what will occur in each of the test tubes. If a change or reaction occurs, write the new chemical formula.
c. Cu2+(aq) is in one test tube and Ag+(aq) is in another test tube. HCl is added to both test tubes. Describe what will occur in each of the test tubes. If a change or reaction occurs, write the new chemical formula.
d. Pb2+(aq) is in one test tube and Bi3+(aq) is in another test tube. HCl is added to both test tubes. Describe what will occur in each of the test tubes. If a change or reaction occurs, write the new chemical formula.
3. a. What reagent could you add to a solution containing a mixture of Cu2+(aq) and Bi3+(aq) to separate the two species?
b. What reagent could you add to a solution containing a mixture of Cu2+(aq) and Pb2+(aq) to separate the two species?
4. a. A white precipitate is present in a test tube, but the test tube is not labeled. It could be AgCl or Bi(OH)3. What reagent could you add to the solid to determine what it is? Describe what will happen to the precipitate when the reagent is added if it is AgCl and what will happen if it is Bi(OH)3.
b. A colorless solution is present in a test tube, but the test tube is not labeled. It is either Pb2+(aq) or Bi3+(aq). What reagent could you add to the solution to determine what it is? Describe what will happen to the solution when the reagent is added if it is Pb2+(aq) and what will happen if it is Bi3+(aq).
c. A white precipitate is present in a test tube, but the test tube is not labeled. It could be AgCl or PbCl2. What reagent could you add to the solid to determine what it is? Describe what will happen to the precipitate when the reagent is added if it is AgCl and what will happen if it is PbCl2.
Activity Completed!