Chapter 1. Experiment 17
Investigating pH and Buffer Properties
Purpose of the Experiment
To look at the relationship between pH and acidic or basic character, and acid–base titration curves.
Background Required
This experiment will use basic laboratory techniques in using a pH meter and buret. The concepts of acids, bases, and pH are used in this experiment.
Background Information
Acids and bases are found everywhere. These compounds are found in our air, water, and soil, the foods we consume, and even inside our bodies! This is one area of chemistry that is applied to many areas of science.
In aqueous solutions, we generally use the Brønsted-Lowry definitions. An acid is any substance that donates a proton, H+. A base is any substance that receives a proton. Compounds that can donate or receive more than one proton are polyprotic acids or polyprotic bases. In water, the H+(aq) ion hydrogen bonds with water to form the hydronium, H3O+ ion. In texts, H+(aq) is used interchangeably with H3O+(aq).
A shorthand calculation is used to indicate the concentration of H+(aq) in solution. It allows low concentrations to be written without use of scientific notation.
pH = – log[H+] or pH = – log[H3O+]
The pH scale in typical aqueous solution ranges from 0–14. (There are some VERY acidic compounds that have a negative pH.) Acidic solutions have a pH < 7 and basic solutions have a pH > 7. Neutral solutions have a pH of 7.
A pH buffer is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffered solution’s pH value doesn’t change much with small additions of acid or base. We often determine how much acid (or base) can be added before the buffer is “overwhelmed” and the pH values increase (or decrease) significantly. The amount of acid (or base) that can be added before the pH value changes by +/–1 pH unit is called its buffering capacity.
A buffer works because it has both a weak acid (HA) and its conjugate base (A–) present. The weak acid species reacts with any added base, and the weak (conjugate) base species reacts with any added acid. And the same is true for a weak base and its conjugate acid.
Neutralize added base (OH–): HA + OH– ⇋ A– + H2O
Neutralize added acid (H+): A– + H3O+ ⇋ HA + H2O
The pH of an acidic buffer solution can be calculated from the weak acid dissociation equilibrium with initial concentrations of both the weak acid and the salt of its conjugate base.
Example 1
Problem
What is the pH of a solution that is 2.8 × 10–3 M H3O+? Is the solution acidic, basic, or neutral?
Solution
Use the definition of pH to calculate the pH and then apply it to the pH scale.
pH = – log[H3O+]
pH = – log(2.8 × 10–3)
pH = 2.55
Since the pH < 7, the solution is acidic.
Example 2
Problem
What is the pH for a buffer that is a mixture of 0.25 M HC2H3O2 and 0.20 M NaC2H3O2?
Solution
As a sodium salt, NaC2H3O2 dissolves 100% into Na+ and C2H3O2–, so the initial concentration of C2H3O2– is 0.20 M.
Use the weak acid dissociation equilibrium of HC2H3O2 and use the initial values of both HC2H3O2 and C2H3O2–.
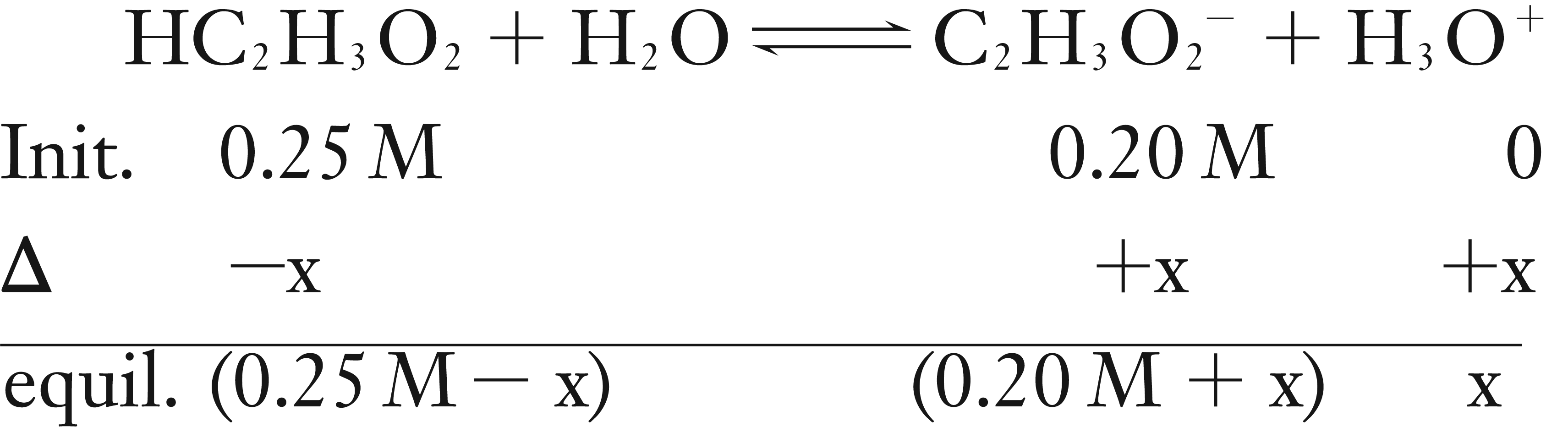
Assume that x is very small in comparison to 0.25 M and 0.20 M, so 0.25 M – x ≈ 0.25 M and 0.20 M + x ≈ 0.25 M.
Solve for [.H3O+]
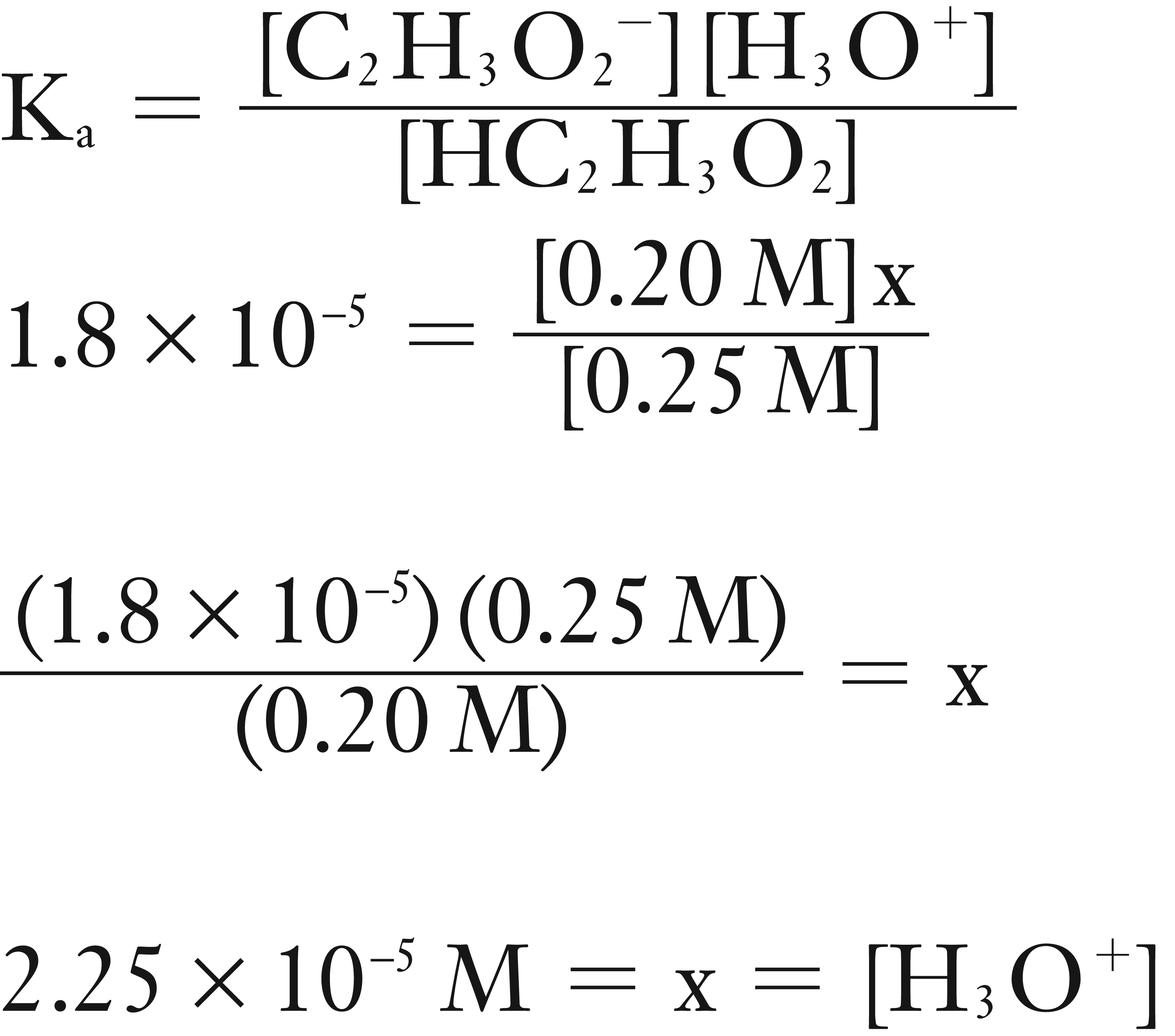
Now solve for pH.
pH = – log [H3O+]
pH = – log (2.25 × 10-5)
pH = 4.65
In This Experiment
In this experiment you will determine the pH of some common chemicals and determine any acidic or basic property trends.
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Determining pH of Common Chemicals and Examining Trends
1. Obtain approximately 5 mL in a small, clean sample vial of each of the 0.1 M common chemicals available in your lab. Label each vial with chemical name.
2. Obtain a LabQuest and pH sensor. Remove the pH sensor from its storage container and connect pH sensor to the LabQuest. The pH readings appear in the pH box (red box) on the METER SCREEN.
3. Rinse the end of the pH probe with a stream of deionized water.
Place the probe in the vial containing one of your chemicals. Record the pH.
Remove the probe and rinse with deionized water.
4. Test and record the pH of each of the remaining common 0.1 M chemicals. Rinse the probe between each solution. Pour all solutions down the drain with lots of running water.
5. Obtain exactly 1.0 mL of 0.1 M HCl in a 10 mL graduated cylinder. Add enough water to the cylinder to get exactly 10.0 mL.
You should add the last mL of water by adding drops with a disposable pipet.
Pour the diluted solution into a large test tube and stir. This is the first dilution. Calculate the Molarity of this dilute solution.
6. Test and record the pH of the diluted HCl solution.
7. Obtain exactly 1.0 mL of the diluted HCl (from Step 5) in a 10 mL graduated cylinder. Add enough water to the cylinder to get exactly 10.0 mL.
Pour into a large test tube and stir. This is the second dilution. Calculate the Molarity of this solution.
8. Test and record the pH of this second dilution HCl solution. Pour the HCl solutions down the drain with lots of running water. Rinse the test tubes with deionized water.
9. Obtain exactly 1.0 mL of 0.1 M NaOH in a 10 mL graduated cylinder. Add enough water to the cylinder to get exactly 10.0 mL.
Pour into a large test tube and stir. This is the first dilution. Calculate the Molarity of this dilute solution.
10. Test and record the pH of the diluted NaOH solution.
11. Obtain exactly 1.0 mL of the diluted NaOH (from Step 9) in a 10 mL graduated cylinder. Add enough water to the cylinder to get exactly 10.0 mL.
Pour into a large test tube and stir. This is the second dilution. Calculate the Molarity of this dilute solution.
12. Test and record the pH of the second dilution NaOH solution. Pour the NaOH solutions down the drain with lots of running water.
Part II
Buffers
13. Obtain about 50 mL of deionized water in a small beaker. You will use this water to rinse the syringe after each use. Rinse a 10 mL syringe with water.
Obtain about 20 mL of 0.10 M HC2H3O2 in a small beaker and label the beaker.
Place the pH probe in the beaker and record the pH.
Obtain about 15 mL of 0.10 M NaC2H3O2 in a small beaker and label the beaker.
Place the pH probe in the beaker and record the pH.
14. Add 15 mL of 0.10 M HC2H3O2 to a 25 mL volumetric flask using the syringe. Rinse the syringe with water.
Add 10 mL of 0.10 NaC2H3O2 to the same volumetric flask using the syringe.
Seal the flask with Parafilm and invert the flask to mix.
Pour the contents of the buffer solution into two clean 100 mL beakers and label each as Buffer 1.
15. Obtain about 15 mL of 0.50 M HC2H3O2 in a small beaker and label the beaker.
Obtain about 20 mL of 0.50 M NaC2H3O2 in a small beaker and label the beaker.
16. Add 10 mL of 0.50 M HC2H3O2 to a 25 mL volumetric flask using the syringe. Rinse the syringe with water.
Add 15 mL of 0.50 M NaC2H3O2 to the same volumetric flask using the syringe.
Seal the flask with Parafilm and invert the flask to mix.
Pour the contents of the buffer solution into two clean 100 mL beakers and label each as Buffer 2.
17. Add 50 mL of deionized water to each beaker.
Place the pH probe in one of the Buffer 1 solutions and record the pH.
Rinse the pH probe with water.
Place the pH probe in one of the Buffer 2 solutions and record the pH.
Rinse the pH probe with water.
Part III
Buffer Capacity
18. Place a buret in each of the buret clamps. Label one buret 1.0 M HCl and label the other buret 1.0 M NaOH.
Rinse each buret with the appropriate 1.0 M solution.
Fill each buret half with the appropriate 1.0 M solution.
19. Place one of the beakers with Buffer 1 under the buret with HCl and the other beaker with Buffer 1 under the buret with NaOH.
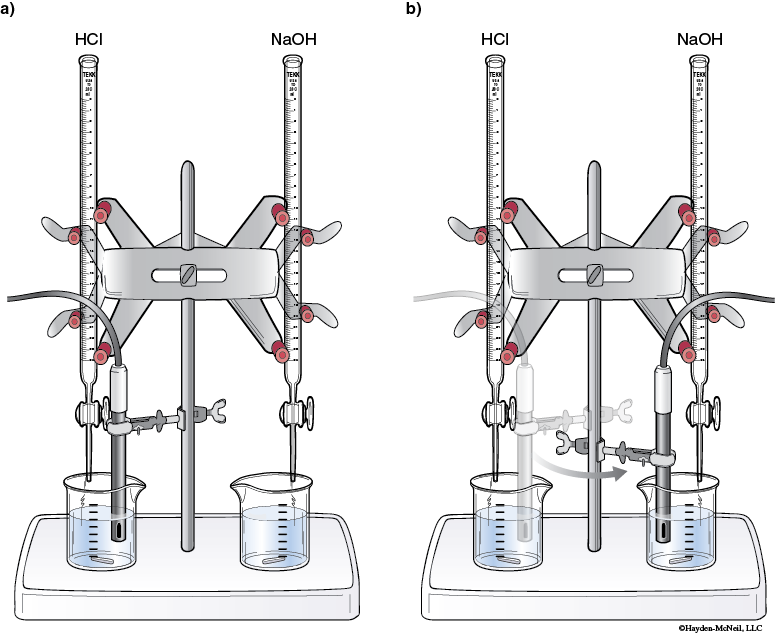
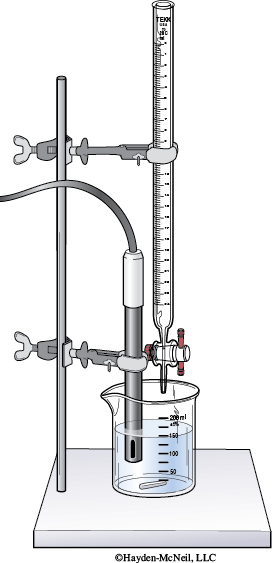
20. Clamp the pH probe (or place in the electrode holder) so that the tip of the probe is in the Buffer 1 solution (on the HCl side) but not touching the bottom of the beaker.
Record the pH of this solution.
Add 0.5 mL of the 1.0 M HCl to the beaker. Swirl the beaker.
Record the pH reading and the volume added.
Repeat adding 0.5 mL of 1.0 M HCl and recording the pH after each addition until the pH is 1 pH unit lower than initial pH reading.
21. Move the pH probe to the beaker under the NaOH buret.
Record the pH of this solution.
Add 0.5 mL of the 1.0 M NaOH to the beaker. Swirl the beaker.
Record the pH reading and the volume added.
Repeat adding 0.5 mL of 1.0 M NaOH and recording the pH after each addition until the pH is 1 pH unit higher than the initial pH reading.
22. Repeat steps 20 and 21 with the two beakers of Buffer 2.
23. Dispose of all solutions down the drain with lots of running water.
Return the pH probe to its screw top vial. If there is not enough storage solution in the vial, take the pH probe and storage vial to the Stockroom to fill the storage vial.
This storage solution is NOT just water.
Determinations
For the common chemicals, classify each as acidic, basic, or neutral.
Create a pH scale of the chemicals (listing chemicals in order of low pH to high pH).
Compare the pH of the 0.1 M HCl solution to the pH of the first and second HCl dilutions. What happens to the pH as an acidic solution becomes diluted?
Compare the pH of the 0.1 M NaOH solution to the pH of the first and second NaOH dilutions. What happens to the pH as a basic solution becomes diluted?
What is the unique characterization of a pH buffer?
Compare the pH readings of acetic acid and the sodium acetate to the pH readings of the buffers.
Which buffer had the greater buffering capacity and why?
Calculate the expected initial pH for Buffer 1 and for Buffer 2.
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
1. In looking at the chemical alert information for the acids and bases, what is the common chemical alert information?
What should a person do if they spill any acid or base on their skin?
2. HCl is a strong acid, that is, it completely dissociates in solution. So a 0.02 M HCl solution really contains 0.02 M H+ and 0.02 M Cl– ions and no HCl.
What is the expected pH of a 0.02 M HCl solution?
3. NaOH is a strong base, that is, it completely dissociates in solution. So a 0.02 M NaOH solution really contains 0.02 M Na+ and 0.02 M OH– ions and no NaOH.
What is the expected pH of a 0.02 M NaOH solution?
4. A student takes 2.0 mL of a 0.20 M NaOH solution and then dilutes it with water to a final volume of 10.0 mL. What is the Molarity of the diluted NaOH solution?
5. HCl is a strong acid, that is, it completely dissociates in solution.
a. What is the expected pH of a 0.1 M solution?
How does this compare to your experimental pH?
b. What is the expected pH of the HCl first dilution solution?
How does it compare to your experimental pH?
c. What is the expected pH of the HCl second dilution solution?
How does it compare to your experimental pH?
6. NaOH is a strong base, that is, it completely dissociates in solution.
a. What is the expected pH of a 0.1 M NaOH solution?
How does this compare to your experimental pH?
b. What is the expected pH of the NaOH first dilution solution?
How does it compare to your experimental pH?
c. What is the expected pH of the NaOH second dilution solution?
How does it compare to your experimental pH?
7. What is meant by buffering capacity of a solution?
8. How does the concentration of the chemicals in a buffer affect the buffer capacity?
9. Calculate the pH of a solution that is 0.30 M HNO2 and 0.45 M NaNO2.
Ka (HNO2) = 4.5 × 10–4
Activity Completed!