Chapter 1. Experiment 21
Calories from Fat in Peanuts and Other Nuts
Purpose of the Experiment
Estimate how many Calories from fat are in peanuts and other nuts.
Background Required
This experiment will use basic laboratory measurements of liquids, solids, and temperature. The concepts of specific heat capacity and calorimetry are used in this experiment.
Background Information
One way to measure the energy of a compound is to measure the heat given off during combustion. The energy is released during the combustion as heat. We use a calorimeter to measure how much heat is released (or absorbed) by combustion. Calorimeters are also used to measure the heats of other reactions besides combustion. Even though the SI unit for energy is the Joule, the older unit of the calorie is still often used. A calorie is defined as the amount of heat energy required to raise the temperature of 1 gram of water by 1°C. Since it is a small unit, most reactions are expressed in kilocalories (1000 cal = 1 kcal). In the food industry, the calories on labels are in fact kilocalories. Often these calories are abbreviated as Cal, to show that these are food calories.
The calorimeter is under constant pressure, so by definition, the heat released (q) is also the enthalpy (energy). The equation used to calculate heat (q) is given below.
q = (specific heat capacity) (mass) (ΔT)
So in calorimetry, the change of temperature of the surroundings (water) is measured directly and qsurroundings is calculated. We assume that all of the heat released (qsystem) by the combustion is absorbed by the water (qsurroundings) in the calorimeter. The specific heat capacity of water is 1.0 cal/g°C and the density of water is 1.00 g/mL.
qsystem = –qsurroundings
In This Experiment
In this experiment, the calorie content from fat for cocktail peanuts and other nuts will be determined using a simple calorimeter. Under the conditions we are using, the combustion will be incomplete, that is, not all of the nut will be consumed. It is the oil or fat that burns easily. Therefore the heat being measured is the combustion of the oil. We can compare the results to the nutrition labels for calories from fat.
Example
Problem
How many Calories (kcal) from fat are in a peanut? During the combustion of one peanut (mass of 0.375 g), the temperature of the 200 mL of water in the calorimeter raised from 25.3°C to 32.8°C. How many Calories (kcal) would be present in one serving of peanuts? (Serving size for peanuts is 28 g.)
Solution
(1) Calculate qH2O from the specific heat, mass of water, and change of temperature of the water. Determine qpeanut from qH2O
qH2O = (specific heat capacityH2O) (massH2O) (ΔTH2O)
qH2O = (1.0 cal/g°C) (200 mL × 1 g/mL) (32.8°C 2 25.3°C)
qH2O = 1.50 × 103 cal or 1.50 kcal
(Note the sign is positive for the endothermic process of water absorbing heat from the system.)
qpeanut = –qH2O
qpeanut = –1.50 kcal or –1.50 Cal
(Note the sign is negative for the exothermic process of the system releasing heat to the surroundings.)
Solution
(2)Determine how many kcal or Cal will be present in one serving (28 g) of peanuts.
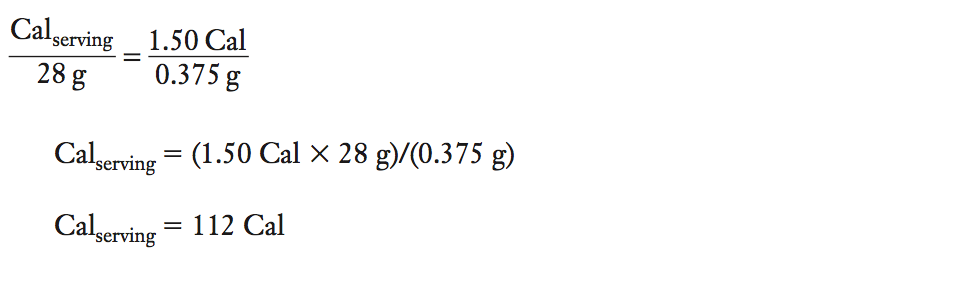
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Setting up the Calorimeter
1. Set up the soft drink can calorimeter as shown in the diagram. Insert the straightened out paper clips through the holes to form a support and place on an iron ring clamped to a ring stand.
2. Form the nut support stand by bending half of a paper clip down and inserting into a cork. (See Figure 21-1.)
3. Lower the iron ring so that the bottom of the can is approximately a thumb’s width from the nut support stand.
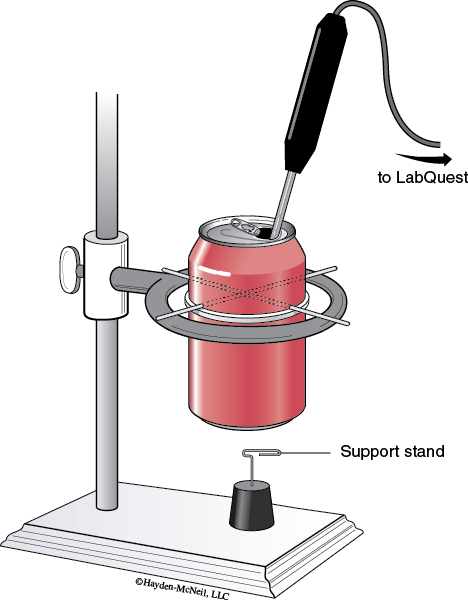
Part II
Combustion of Peanuts
4. Pour 200 mL of water into the can. Place the temperature probe connected to a LabQuest in the drinking hole of the can. If necessary, clamp the probe in place.
5. Determine the mass of a whole peanut (or two halves) using the weighing by difference technique. Record the mass.
6. On the METER SCREEN tap the MODE box. On the MODE SCREEN, keep the mode as time based and adjust the time length to 180 seconds. Click OK.
7. Place the peanut on the support stand. Ignite the peanut using a kitchen match.
It may take 2 or 3 matches to get the peanut to remain lit.
Once burning, start data collection by tapping the PLAY button (green triangle).
8. When the peanut stops burning, swirl the can.
At the end of 3 minutes, tap Analyze at the top of the screen and then select Statistics. Tap the check box for Temperature. Highlight the data. Click OK.
9. Record the min temp as Tinit and max temp as Tfinal.
10. Repeat the procedure with a second peanut. Pour out the water.
You must obtain new water for each trial.
Part III
Combustion of Other Nuts
11. Repeat the procedure twice with one of the other nuts available in your lab.
BE SURE TO WASH YOUR HANDS BEFORE LEAVING THE LAB!
Data Analysis
Calculations & Determination
For each peanut trial, calculate the Cal from fat.
For each peanut, calculate how many Cal would be present in a serving.
(Average serving is 28 g of peanuts). Average your two results.
Next, calculate percent error for your results given that the Nutrition Label states 120 Cal (from fat) per serving.
For each other nut trial, calculate the Cal from fat.
For each nut, calculate how many Cal would be present in a serving.
(Average serving will be given). Average your two results.
Next, calculate percent error for your results.
(Nutrition Label information will be given.)
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results? Do the results seem reasonable?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
Study Questions
1. For each part of the experiment, match each word to its calorimetry function name.
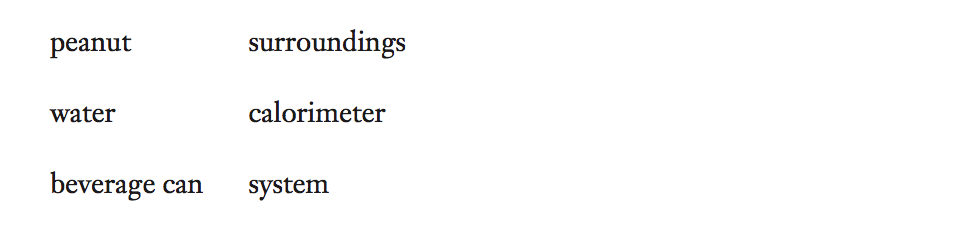
2. During the combustion of a 0.28 g Spanish peanut it raised the temperature of 200 mL of water by 5.9°C. The Nutrition Label states the average serving size of Spanish peanuts is 38 g.
a. How many Cal from fat?
b. How many Cal from fat in a serving?
3. It was assumed that all of the heat given off by the combustion was absorbed by the water.
a. Is this a good assumption?
b. What evidence do you have to support your answer?
c. What effect will this have on the calculated Cal?
4. In an experiment by P. Nutt, one peanut only burned for 1 minute, while the other peanut had burned for 2 minutes. What effect will this have on the calculated results? (Higher or lower Cal from fat?)
5. P. Nutt asks if the build up of soot affects the results. How would you answer?
6. P. Nutt also asks if the experiment could be done using a Styrofoam cup instead of beverage can. How would you answer and explain your answer?
Activity Completed!