Chapter 1. Experiment 2
Classifying Reactions in the Copper Cycle
Purpose of the Experiment
Investigate different classifications of reactions through a series of reactions that begin and end with Cu(s).
Background Required
This experiment will use basic laboratory techniques of measuring and dispensing volume by a syringe. The concepts of stoichiometry and classifying reactions are used in this experiment.
Background Information
Most reactions can be grouped into one of five general classifications of reactions. The general classifications are described below with both a generic reaction and an example of a specific reaction(s).
Combinations: A + B → AB
2 Mg(s) + O2(g) → 2 MgO(s)
Two elements or species come together to form one compound.
Decomposition: ABC → AB + C
CaCO3(s) → CaO(s) + CO2(g)
One species decomposes into two or more chemical species, generally as a result of heating.
Single Displacement: AC + B → A + BC
Cd(NO3)2(aq) + Zn(s) → Zn(NO3)2(aq) + Cd(s)
One element (B) displaces an atom (A) in a compound (AC) to form a new compound (BC), while the displaced atom (A) is converted to its elemental form.
Double Displacement: AC + BD → AD + BC
Cd(NO3)2(aq) + (NH4)2S(aq) → CdS(s) + 2 NH4NO3(aq)
Zn(OH)2(s) + 2 HCl(aq) → ZnCl2(aq) + 2 H2O(ℓ)
The cation of the second species displaces the cation of the first species to form to new compounds. The two cations are exchanged. This type of reaction is also called a metathesis reaction.
Oxidation-Reduction: Two half-reactions added together.
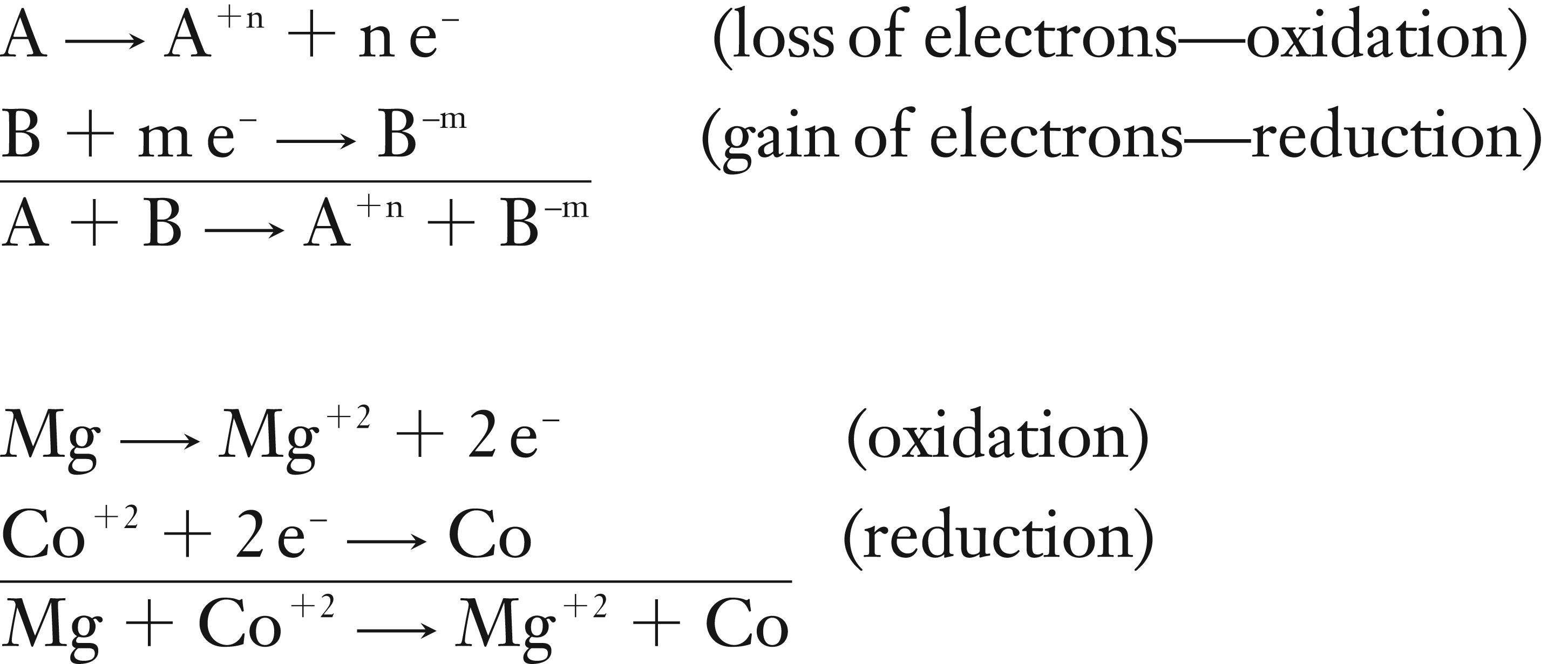
In oxidation half reaction, one species (A) will lose electrons. Then these electrons are used in the reduction half reaction, where the other species (B) gains electrons. The number of electrons transferred must be the same in each half reaction.
In This Experiment
In this experiment, a series of reactions will illustrate these general reactions. Several of the reactions will be done in sequence, that is, the product of the first reaction becomes a reactant in the next reaction. We will start with metallic copper and ultimately end up metallic copper as our final product.
In Part I, the first reaction dissolves the Cu(s) using hydrogen peroxide in a strongly acidic (H2SO4) solution. The reaction is an example of an oxidation-reduction reaction. The reaction is shown as the two half reactions, then as a net-ionic equation. The last reaction is shown as the complete molecular equation.
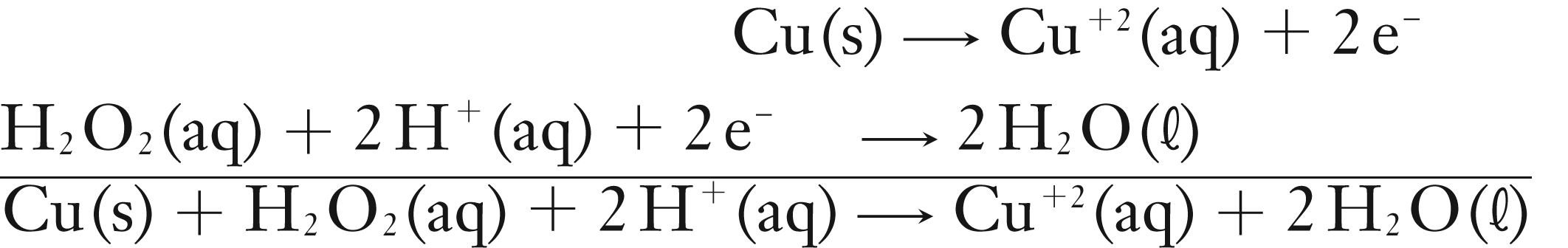
Molecular eqn: Cu(s) + H2O2(aq) + H2SO4(aq) → CuSO4(aq) + 2 H2O(ℓ)
In Part II, the second reaction precipitates the Cu+2 ion as the copper(II) hydroxide, Cu(OH)2(s). This is an example of a double displacement reaction. The Cu+2 ion and the Na+ ions exchange places. However, it takes two Na+ ions to balance the -2 charge of the SO4–2 ion.
Molecular eqn: CuSO4(aq) + 2 NaOH(aq) → Cu(OH)2(s) + Na2SO4(aq)
In Part III, the copper(II) hydroxide from Part II is heated to break down into copper(II) oxide and water. This is an example of a decomposition reaction of one chemical species breaking down to form 2 different products.
Molecular eqn: Cu(OH)2(s) → CuO(s) + H2O(ℓ)
In Part IV, another example of a double displacement reaction occurs. Here the copper(II) oxide from Part III is dissolved in sulfuric acid to produce copper(II) sulfate and water. Here the Cu+2 ion and the H+ ions exchange places.
Molecular eqn: CuO(s) + H2SO4(aq) → CuSO4(aq) + H2O(ℓ)
In Part V, the solid copper is produced when the more active metal, Zn, is added to the CuSO4 solution from Part I. This is an example of a single displacement reaction. The element Zn displaces the Cu+2 ion to become ZnSO4 while the Cu+2 becomes Cu(s). As is the case with most single displacement reactions, it is also an oxidation-reduction reaction. The Zn(s) loses 2 e– to form Zn+2 and the Cu+2 ion gains 2 e– to form Cu(s).
Molecular eqn: CuSO4(aq) + Zn(s) → Cu(s) + ZnSO4(aq)
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
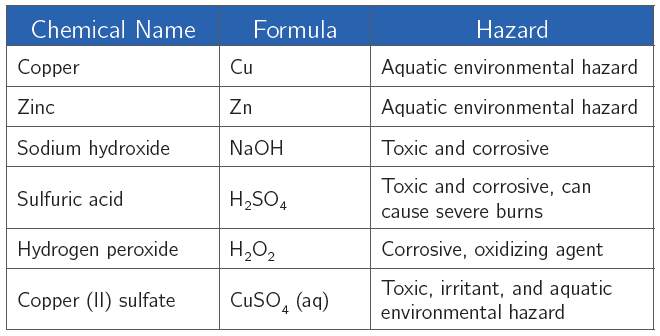
Chemical Alert:
Part I
Dissolving the Cu(s)
1. Prepare a hot water bath by adding about 150 mL of hot tap water into a 400 mL beaker. Place the beaker on a hot plate and bring to a low boil.
2. Add one micro-scoop of Cu turnings to a clean small test tube.
Label the test tube as “A” and place in the test tube rack.
3. Fill a 50 mL beaker about half full with water. Label the beaker as “H2O.”
Rinse a plastic 5 mL graduated syringe by placing the tip of the syringe in the water. Pull back on the plunger to pull up 5 mL of water, then push plunger down to expel the water into the sink.
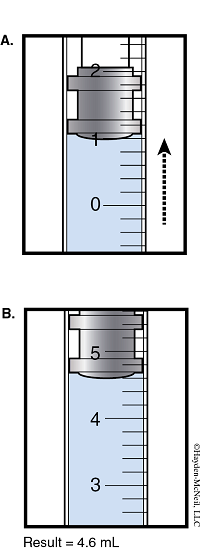
4.Obtain approximately 5 mL of the H2O2–H2SO4 solution in a second 50 mL beaker.
If the H2O2–H2SO4 mixture gets on your skin, wash immediately with cold water for 10 minutes.
5.Use the syringe to obtain 1 mL of the H2O2–H2SO4 mixture.
Slowly push the plunger so that it dispenses 1 drop at a time into test tube “A.”
Record your observations.
Discard the H2O2–H2SO4 solution by pouring down the drain with lots of running water.
Rinse this beaker with tap water. Rinse the syringe with water from the beaker.
6.When all the Cu is dissolved, place the test tube “A” in the hot water bath.
The excess H2O2 will decompose with heat to form water and O2(g).
After about 5–7 minutes (the bubbles should cease), remove the test tube from the water bath and place it in the test tube rack to cool.
Go on to the next steps while the test tube is cooling. Test tube “A” now contains an aqueous solution of CuSO4, which will be used later in the experiment.
Part II
Precipitating Cu(OH)2(s)
7. Label a second clean test tube “B.” Obtain about 5 mL of 0.2 M CuSO4 solution in a clean 50 mL beaker.
8. Using the 5 mL syringe (which you previously rinsed), draw up 2 mL of solution and add the 2 mL to test tube “B.”
9. Slowly add 5 drops of 6 M NaOH to the test tube, stirring constantly.
Record your observations. This solution contains Cu(OH)2(s).
Part III
Forming CuO(s)
10. Place test tube “B” in the hot water bath for 3 minutes.
Record your observations. Return the test tube to the test tube rack.
11. After a few minutes, hold the test tube in the water beaker to finish cooling the test tube down.
12. Remove and place the test tube back in the test tube rack. This solution contains CuO(s).
Part IV
Dissolving the CuO(s)
13.Add 5 drops of 6 M H2SO4 to test tube “B.”
14.Stir continuously for 1 minute.
Record your observations. Set this test tube aside.
This solution contains CuSO4(aq).
Part V
Reforming Cu(s)
15. Obtain a micro-scoop of Zn metal and add it the original test tube “A.”
Occasionally stir the solution.
16. After 5 minutes, record your observations.
The reaction is complete when the solution is colorless.
17. Place two pieces of large filter paper on a watch glass.
Swirl the contents of test tube “A” and with a quick motion, pour it onto the filter paper.
18. Observe the solid that was produced and compare to the original Cu(s).
19. Discard all solutions in the waste container in the hood.
Scrape the copper solid into the waste jar in the hood and discard the filter paper in the trash can.
Wash all glassware. Rinse the syringe and take it back to the dispensing area.
Data Analysis & Discussion
Determinations
Make a table with each Cu species that was observed and indicate its color and physical state.
Write all the reactions and classify each as the general type of reaction.
Study Questions
1. Write the generic reaction for each of the five general classifications of reactions.
2. Which chemicals used in this experiment are corrosive? What should you do if some of these chemicals come into contact with your skin?
3. If some red-brown Cu(s) is heated with a Bunsen burner in the presence of O2(g), some black solid, copper(II) oxide, CuO(s), forms. Write the balanced equation for this reaction. Classify this reaction as one of the five general types.
4. Penny and Benedict used 0.510 g of Cu(s) in the initial reaction of this experiment. At the end of the experiment, they had obtained 0.384 g of Cu.
a. What is their theoretical yield?
b. What is their actual yield?
c. What is their percent yield?
Activity Completed!