Chapter 1. Experiment 5
Citric Acid in Fruit Juices
Purpose of the Experiment
Estimate how much citric acid is present in a fruit juice.
Background Required
This experiment will use basic laboratory techniques of titration. The concepts of acid–base neutralization reactions and stoichiometry are used in this experiment.
Background Information
Acid comes from the Latin word acidus, which means sour or sharp. One characteristic of acids is their sour taste. The sour or tart taste of fruit juice is primarily due to citric acid, H3C6H5O7, a triprotic acid (3 acidic protons). In this experiment, it is assumed that all the acid present in the fruit juice is citric acid. To determine how much citric acid is present, the citric acid is neutralized by a solution of NaOH in a titration.
H3C6H5O7(aq) + 3 NaOH(aq) → Na3C6H5O7(aq) + 3 H2O(ℓ)
Titration is a method to analyze how much analyte (species of interest) is present. In a titration, the titrant (species being added) is added in small increments to the analyte solution until the reaction is complete. By determining how many moles of titrant were needed to react with the analyte, by stoichiometry one can determine how much analyte was present. To signal the end of the reaction, an acid–base indicator (a species that changes colors at different pHs) is used. The indicator will change colors when the pH matches the end of the reaction. For this reaction, phenolphthalein is the acid–base indicator. It is colorless at acidic pHs (beginning of the reaction) and turns bright pink at basic pHs (end of the reaction).
In This Experiment
In this experiment, the citric acid (analyte) in the fruit juice is being titrated with NaOH (titrant) to the phenolphthalein (indicator) end point. The mg of citric acid per mL of fruit juice will be determined. Some good lab skills for titrations are reviewed here.
Rinse buret: Rinse the buret by adding about 5 mL of the titrant (NaOH) to the buret. Let some of the solution out by turning the stopcock of the buret. Then holding the buret horizontally, roll the buret back and forth in your hands to coat the inside of the buret. Finally pour the solution out.
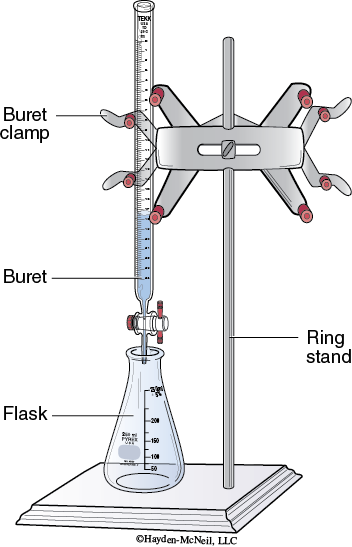
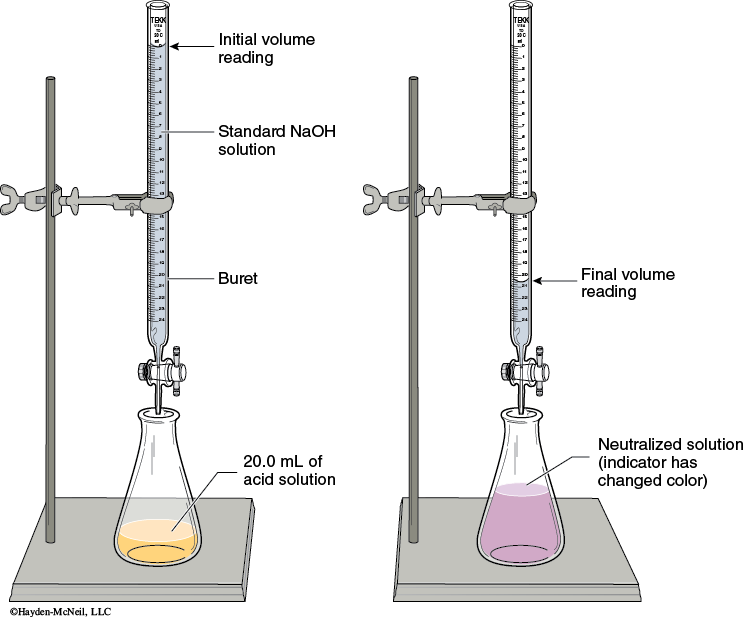
Setup: Assemble the apparatus for titration as shown in Figure 5-1.
Eliminating bubbles: Use a funnel in the top of the buret to add the titrant to the buret. Pour enough titrant to fill the buret past zero. Replace the flask with a beaker. Turn the stopcock to drain some of the titrant. There may be bubbles in the tip of the buret. Turning the stopcock back and forth to drain some titrant will generally force the bubbles out.
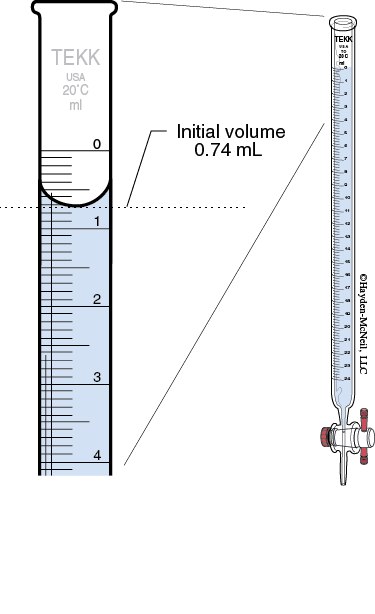
Reading the buret: Read the meniscus at eye level. (You may have to lower the buret in order to get it at eye level.) You will read the volumes to 2 digits past the decimal. This will require you to estimate the last digit. The volume delivered will be the difference between the initial and final buret readings. Never start the titration as zero. This is considered a number bias and takes more effort to exactly drain the titrant to get it exactly at 0.00. The initial volume should be between 0–1 mL.
Titrating: As you titrate, swirl the flask to mix the added titrant with the analyte solution. Initially you will be able to allow the titrant to drain quickly into the flask. When you get close to the end point, the end point color appears, but disappears after swirling the solution. At that point, you need to add the titrant dropwise, swirling after every drop. The end point is when the end point color persists for 30 seconds.
Cleaning buret: When you have finished all your titrations, then drain all of the titrant out of the buret. Rinse the buret out with distilled water, and draining several mL of water out of the tip. Drain all of the water out and return to the appropriate space. Otherwise the chemicals evaporate in the tip and clog the tip.
Example
Problem
How many milligrams of H3C6H5O7 are present per milliliter of juice if 15.00 mL sample of lemon juice required 34.28 mL of 0.1065 M NaOH to reach the end point?
Solution
(1) You need the calculate the mass of H3C6H5O7 using the M and mL of NaOH, stoichiometry and balanced equation.
Convert the mass of H3C6H5O7 to mg and then divide by the mL of juice (analyte).
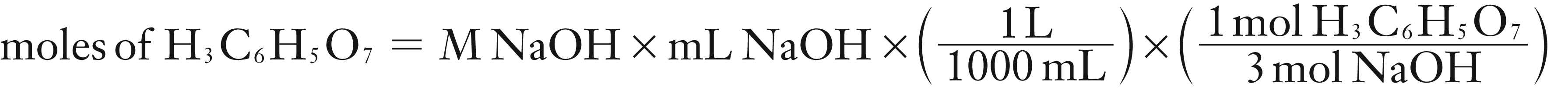
moles of H3C6H5O7 = 1.21694 × 10–3 mol (keep more significant digits until final answer)
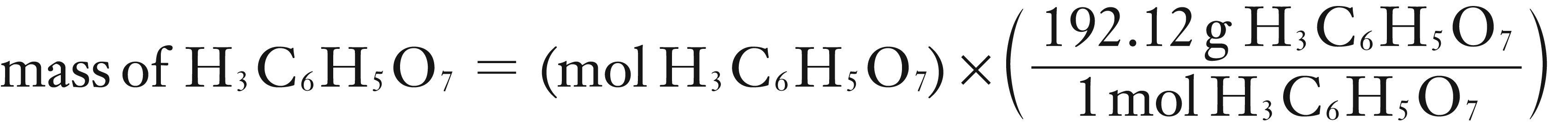
mass of H3C6H5O7 = 0.2337985 g (keep more significant digits until final answer)
(2) You need to calculate the mg of H3C6H5O7 per mL of juice.
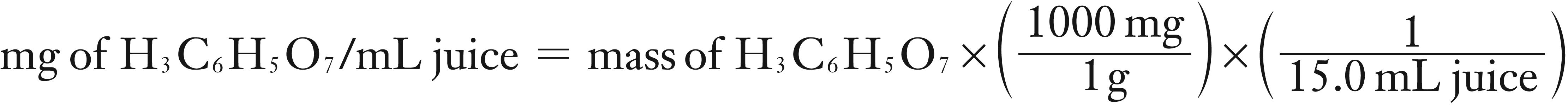
mg of H3C6H5O7 /mL juice = 15.5866 mg/mL rounds to 15.6 mg/mL
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
Chemical Alert:
Part I
Setting Up the Titration
1. Obtain approximately 50 mL of the standardized 0.1 M NaOH solution. The exact molarity of the NaOH solution has been determined for you. Record the exact molarity of the NaOH (titrant).
2. Using some of the NaOH, rinse the buret and then set up the buret for titration. Fill the buret with the NaOH (titrant) and then eliminate any bubbles in the tip of the buret. Drain some of the titrant out into an extra beaker and record the initial volume of NaOH.
Part II
Titrating the Fruit Juice
3. Obtain about 45 mL of the fruit juice (analyte) in a beaker. Transfer 20.0 mL of the juice to a clean 125 mL Erlenmeyer flask. Add 3 drops of phenolphthalein (indicator) to the flask.
4. Titrate the fruit juice with the NaOH. Slow the titrant flow to dropwise when some pink color is observed in the solution. The titration is complete when the end point is reached.
For phenolphthalein, the end point is observed when the faint pink color persists for half a minute.
Record the final volume of the NaOH.
If the solution is a dark pink color, then you have overtitrated (added too much titrant). This is a titration error.
5. Transfer 20.0 mL of the juice to a second 125 mL Erlenmeyer flask. Add 3 drops of phenolphthalein to the flask.
6. Refill the buret with NaOH and record the initial volume of NaOH.
Titrate the fruit juice solution with the NaOH to the end point. Record the final volume.
7. Pour all solutions down the drain with lots of running water. Drain out all the remaining titrant. Rinse out buret with water, allowing water to drain through the tip.
Calculations
For each trial, calculate the mass of citric acid present in the fruit juice sample.
Next, calculate the mg of citric acid present per mL of juice.
Finally, average the mg of H3C6H5O7 /mL juice from each titration trial.
Discussion
1. Summarize the results of this experiment.
What did you set out to determine in this experiment, what is the average mg H3C6H5O7 /mL juice?
2. What is the quality of the results? Do the results seem reasonable?
Were the results from each trial close to each other (precision)? Most fruit juices contain anywhere from 5–50 mg H3C6H5O7 /mL juice. Is your juice within that range?
3. What were the errors or possible errors in the experiment?
List any errors or problems you encountered in the procedure. Example: Did you overtitrate (add too much titrant) and get the dark pink color? Did you have trouble observing the color changes? What possible errors could have been encountered?
4. How would the errors affect the results of the experiment?
Example: If you overtitrated the sample, then the volume of titrant used in the calculations will be too large. How will this affect the calculation of mg H3C6H5O7 /mL juice? Will it make the number bigger or smaller than it should be?
Study Questions
1. For each chemical match it to its function name and where you will find it in the experiment.
2. How will you recognize the end point of the titration? (What is observed that signals to stop adding the titrant?)
3. Two students titrated a 25.0 mL aliquot of pear juice with 0.107 M NaOH to the phenolphthalein end point. The initial buret reading was 0.73 mL and the final buret reading was 18.39 mL.
a. What is the mass of citric acid in the juice sample?
b. Calculate the mg of citric acid present per mL of juice.
4. Titrations are a type of stoichiometry calculation. In this experiment what is the mole ratio between citric acid and NaOH?
5. How would the following errors affect the calculation of mg of citric acid present per mL of juice? Will it make the values too large or too small or no effect? Explain your answers.
a. Overtitrating the end point.
b. Stop titrating at the first sign of pink in the solution.
c. Recording that 20.0 mL of juice was titrated, when actually only 10 mL of juice was present.
d. The Molarity of NaOH was recorded as 0.180 M NaOH, instead of the actual molarity of 0.109 M NaOH.
6. If the juice to be titrated was yellow, the end point color would change from the pink to more of a peach (pink of the indicator at the end point + yellow solution = peach color). Would you be able to titrate purple grape juice by this technique?
7. In the introduction, we stated that other acids are present in fruit juices, but we assumed that the only acid that we were titrating was citric acid. Does this assumption make our results of mg of citric acid present per mL of juice high or low? Explain.
Activity Completed!