Chapter 1. Experiment 7
Investigating Conductivities of Aqueous Solutions and Conductimetric Titration
Purpose of the Experiment
To investigate the conductivities of different aqueous solutions and to determine the concentration of Ba(OH)2 solution by conductimetric titration.
Background Required
This experiment will use basic laboratory techniques using a LabQuest conductivity probe and a buret. The concepts of strong, weak and nonelectrolytes are used in this experiment. Calculations are based on titration concepts.
Background Information
The ability of an aqueous solution to conduct electricity is one way to classify compounds and to obtain a better understanding of how compounds exist in an aqueous solution. To be able to conduct electricity, the solution must contain ions. The more ions present in solution, the better the conductivity of the solution will be. A nonelectrolyte is any substance that when dissolved in water stays as a molecule (doesn’t form ions) and does not conduct electricity. A weak electrolyte is any substance that when dissolved in water partially dissociates into ions, so a few ions are present and it conducts electricity weakly. A strong electrolyte is any substance that when dissolved in water totally dissociates to form many ions, and conducts electricity strongly. Generally, we will compare solutions with the same concentration so that the conductivity comparisons are based on type of substance, not concentrations.
Pure water does not contain ions in an appreciable amount, so it is a nonelectrolyte. However, tap water does have dissolved minerals and ions present, so it is a weak electrolyte.
When the conductivity probe is placed in a solution containing ions, an electrical circuit is completed across the two electrodes found on either side of the hole at the bottom of the probe, thus producing a conductivity reading. The unit of conductivity used is microsiemens per centimeter, µS/cm.
The concentration of an unknown solution can be determined by monitoring the conductivity of the solution during the reaction. In the following reaction, Ba(OH)2 and H2SO4 are strong electrolytes (dissociate completely), but BaSO4 (an insoluble solid) and H2O(ℓ) are nonelectrolytes.
Molecular Equation
Ba(OH)2(aq) + H2SO4(aq) → BaSO4(s) + 2 H2O(ℓ)
Net Ionic Equation
Ba+2(aq) + 2 OH–(aq) + 2 H+(aq) + SO4–2(aq) → BaSO4(s) + 2 H2O(ℓ)
Initially the Ba(OH)2 solution will have a high conductivity. As H2SO4 is added, it reacts with the Ba+2 and OH– ions to form the insoluble BaSO4 and H2O, thus decreasing the amount of ions in solution. So as the titration proceeds, the conductivity will decrease. At the equivalence point, there are equal moles of the two reactants, this solution will contain only products (no ions present). So for this reaction, the conductivity will be close to zero at the equivalence point. After the equivalence point, the conductivity will increase again as excess H+ and SO4–2 ions are added to the solution.
Using the known Molarity and the equivalence point volume of H2SO4, the moles of H2SO4 are calculated. From the mole:mole ratio in the balanced equation, the moles of Ba(OH)2 are determined, and finally the Molarity of Ba(OH)2 is calculated from the moles and the initial volume of Ba(OH)2.
Example
Problem
Classify the following solutions based on their conductivities below.
0.5 M ethylene glycol—20 µS/cm
0.5 M Ca(NO3)2—9143 µS/cm
0.5 M HClO—325 µS/cm
Solution
Use the definitions of electrolytes.
0.5 M ethylene glycol—nonconducting solution, nonelectrolyte
0.5 M Ca(NO3)2—highly conducting solution, strong electrolyte
0.5 M HClO—weakly conducting solution, weak electrolyte
Example
Problem
What is the Molarity of a Ba(OH)2 solution that took 8.20 mL of 0.100 M H2SO4 solution to reach the equivalence point when titrating 10.0 mL of the Ba(OH)2 solution? (See balanced reaction above.)
Solution
Use the M and mL of H2SO4 and stoichiometry to find moles of Ba(OH)2. Then divide the moles of Ba(OH)2 by the L of initial Ba(OH)2 to find M.
In This Experiment
In this experiment you will determine the conductivities of some common chemicals and classify them as nonelectrolytes, weak electrolytes, or strong electrolytes. Then you will monitor the titration of Ba(OH)2 (analyte) with H2SO4 (titrant) to determine the equivalence point in this conductimetric titration.
Procedure
Always Wear Safety Goggles and Use Good Lab Practices
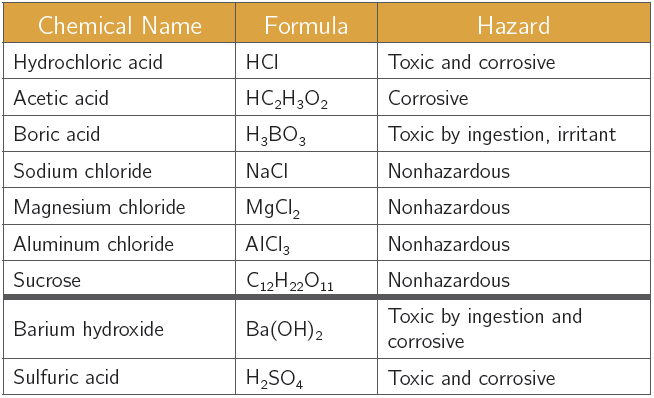
Chemical Alert:
Part I
Determining Conductivities of Common Chemicals
1. Obtain ~5 mL in a small, clean sample vial of each of the first seven chemicals (0.050 M). Label each vial with chemical name.
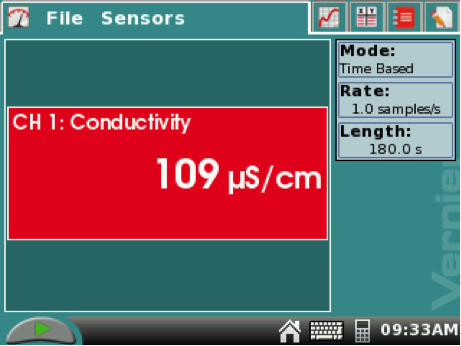
2. Obtain a LabQuest and conductivity probe. Find the switch on the black box and switch to range 0–20,000 µS. Connect the conductivity probe to the LabQuest. The conductivity readings will appear in the red box on the METER SCREEN.
Do not touch or try to dry the end of the rod in the probe. HANDLE WITH CARE!
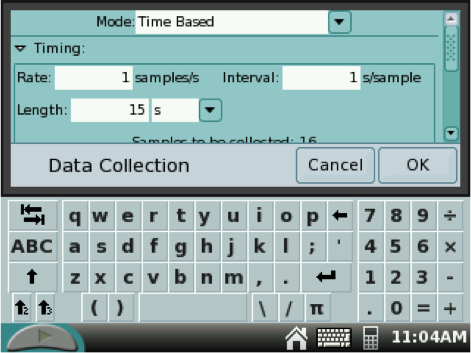
3. Using the stylus, tap the box on the right side with the time (usually set at 180 seconds) as seen in the first figure. In the new box, adjust the time length to 15 seconds. Tap OK when finished to go back to the METER SCREEN.
4. Rinse the end of the conductivity probe with a stream of deionized water.
Place the probe in the vial containing one of the chemicals.
Begin data collection by tapping the PLAY button (green triangle) on the LabQuest’s METER SCREEN.
5. When the data stops, it automatically resizes the graph (see example below). Drag the stylus over the 15-second increment. (This highlights the selected data.) Tap ANALYZE at the top of the screen, select STATISTICS, then check the CONDUCTIVITY box to find the mean value of conductivity and record this number. (In the example below, the mean was 1419 µS/cm.)
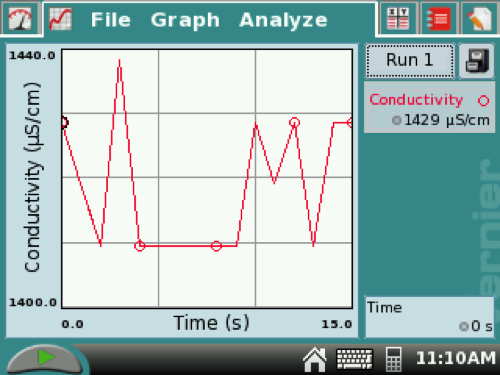
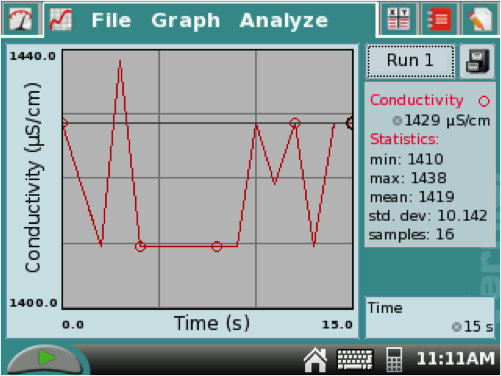
You can resize the graph by tapping Graph, then Graph Options, then click Manual, and scroll down to input a new top (2000) and bottom (0) values. Then click OK. See examples below.
6. Repeat steps 4 and 5 with each of the remaining 0.050 M chemicals.
Discard old run.
Be sure to rinse the probe between each solution with deionized water.
Pour all solutions down the drain with lots of running water.
Part II
Conductimetric Titration
7. Rinse a 25 mL buret with 0.100 M H2SO4 solution and then fill the buret. Attach the buret to the ring stand using a buret clamp. Use a clamp or electrode holder to hold the conductivity probe.
Record the exact Molarity of the H2SO4 solution.
8. Obtain ~15 mL of a barium hydroxide solution. Use a syringe to transfer 10 mL of the solution into a 250 mL beaker. Add 100 mL of distilled water to the beaker. Position the conductivity probe in the solution so that it is not touching the bottom of the beaker.
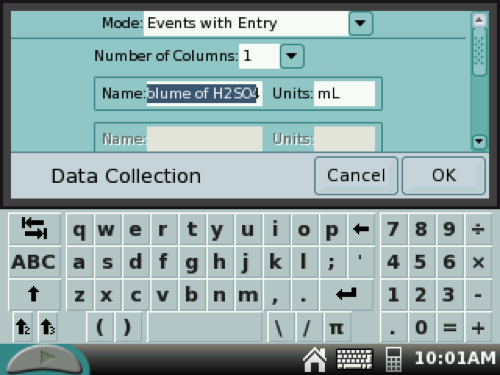
9. Using the stylus, tap the MODE box on the LabQuest. Then select Events with Entry from the pull-down menu.
Type in Volume of H2SO4 in the NAME box and mL in the UNITS box.
Scroll down and tap the check box for Average over 10 seconds. Tap OK.
10. Determine an initial conductivity reading before adding any H2SO4 solution.
Tap the PLAY button (green triangle) to start collecting data. This switches to the GRAPH SCREEN.
Tap the KEEP button (blue square). (It will do a 10-second countdown and average the values.) A prompt screen will appear to enter the volume of H2SO4 added. For initial conductivity (no H2SO4 added), enter “zero” and OK.
Record your initial buret reading on your notebook pages. You will see the first point on the graph.
11. Add 0.5 mL of H2SO4 to the solution and read the buret. Swirl the solution. (A fine white precipitate forms.)
Tap the KEEP button (blue square) to start collecting data. After it averages the reading, enter volume dispensed in the prompt screen.
Volume dispensed = current buret reading – initial buret reading.
12. Repeat step 11, adding ~0.5 mL of H2SO4 and swirl. (Each time, the volume of H2SO4 is current buret reading – initial buret reading.)
13. The conductivity values will decrease as the reaction proceeds. After the equivalence point has been reached, the conductivity values will increase again.
Stop adding H2SO4 after 10 consecutive increasing conductivity values have been obtained. Tap STOP (red square).
If you didn’t record the conductivities for each buret reading, tap the DATA tab (table icon) at the top to see the DATA TABLE screen. Record all the volumes and corresponding conductivity values onto the notebook pages.
14. Sketch your titration in your lab notebook from the GRAPH screen. It should be similar to the first figure below.
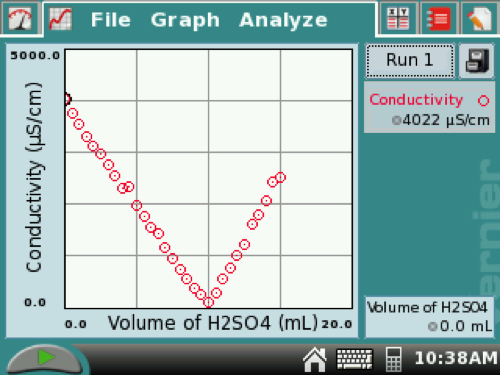
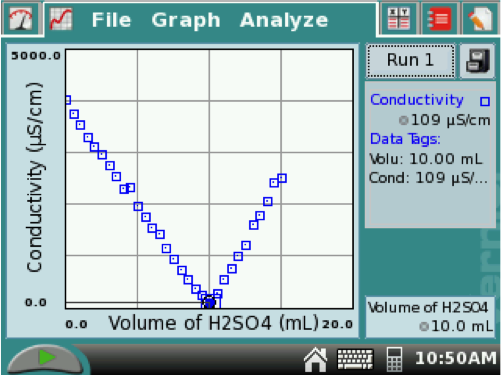
For conductimetric titrations, the equivalence point is the intersection of the two line segments. You can tap the point where the two segments intersect, and the equivalence point volume will be the volume in the lower box. For this example on the right, it was at 10.0 mL.
15. Pour the reaction mixture into the appropriate waste container in the hood.
Rinse the conductivity probe with distilled water and disconnect from the LabQuest. Return the conductivity probe to its storage box and return it and the LabQuest.
Data Analysis
Calculations & Determination
For the common chemicals, classify each as nonelectrolyte, weak electrolyte, or strong electrolyte.
Find the equivalence point on the titration graph. The volume at the equivalence point is the equivalent volume of H2SO4. Determine the Molarity of the Ba(OH)2 solution.
Discussion
1. Summarize the results of this experiment.
2. What is the quality of the results?
3. What were the errors or possible errors in the experiment?
4. How would the errors affect the results of the experiment?
Study Questions
1. An unknown compound X dissolves completely in water and dissociates completely into ions. Which conductivity classification do you predict for X?
2. An unknown compound Y dissolves completely in water and partially dissociates into ions. Which conductivity classification do you predict for Y?
3. An unknown compound Z dissolves completely in water, yet does not dissociates into ions. Which conductivity classification do you predict for Z?
4. Define nonelectrolytes, weak electrolytes, and strong electrolytes in terms of
a. number of ions present in solution.
b. ion dissociation in solution.
c. molecular compounds or soluble ionic compounds.
5. According to the solubility rules for common ionic compounds, all ionic compounds containing Na+ are soluble and dissociate 100%.
What electrolyte classification do you predict for Na+ compounds?
How does this compare to your experimental data with NaCl?
6. Compounds containing only non-metal atoms are generally considered as molecular compounds. An example would be CH3OH.
What electrolyte classification do you predict for molecular compounds?
How does this compare to your experimental data with C12H22O11?
7. Acids that are classified as strong acids (HX), are strong electrolytes because they dissociate 100% in water to form H+ and X–.
What electrolyte classification do you predict for HNO3?
How does this prediction compare to your experimental data with HCl?
8. In a similar conductimetric titration, 7.00 mL of an unknown M of NaOH was titrated with 0.115 M HCl. The equivalence point volume was 12.5 mL of HCl.
a. Complete the titration reaction: NaOH(aq) 1 HCl(aq) → + ?
b. Classify the reactants in terms of electrolytes.
c. Classify the products in terms of electrolytes.
d. As the reaction progresses, will the conductivity go up or down?
As products are made and reactants used up, will you have more or fewer ions in solution?
e. After the equivalence point, when excess HCl is added, will the conductivity go up or down?
f. What is the Molarity of the NaOH?
Activity Completed!