2.5 Threats to the Developing Baby
46
In this section, we’ll explore the prenatal reasons for birth defects, or health problems at birth. I’ll also discuss new research exploring how wider-
Threats from Outside: Teratogens
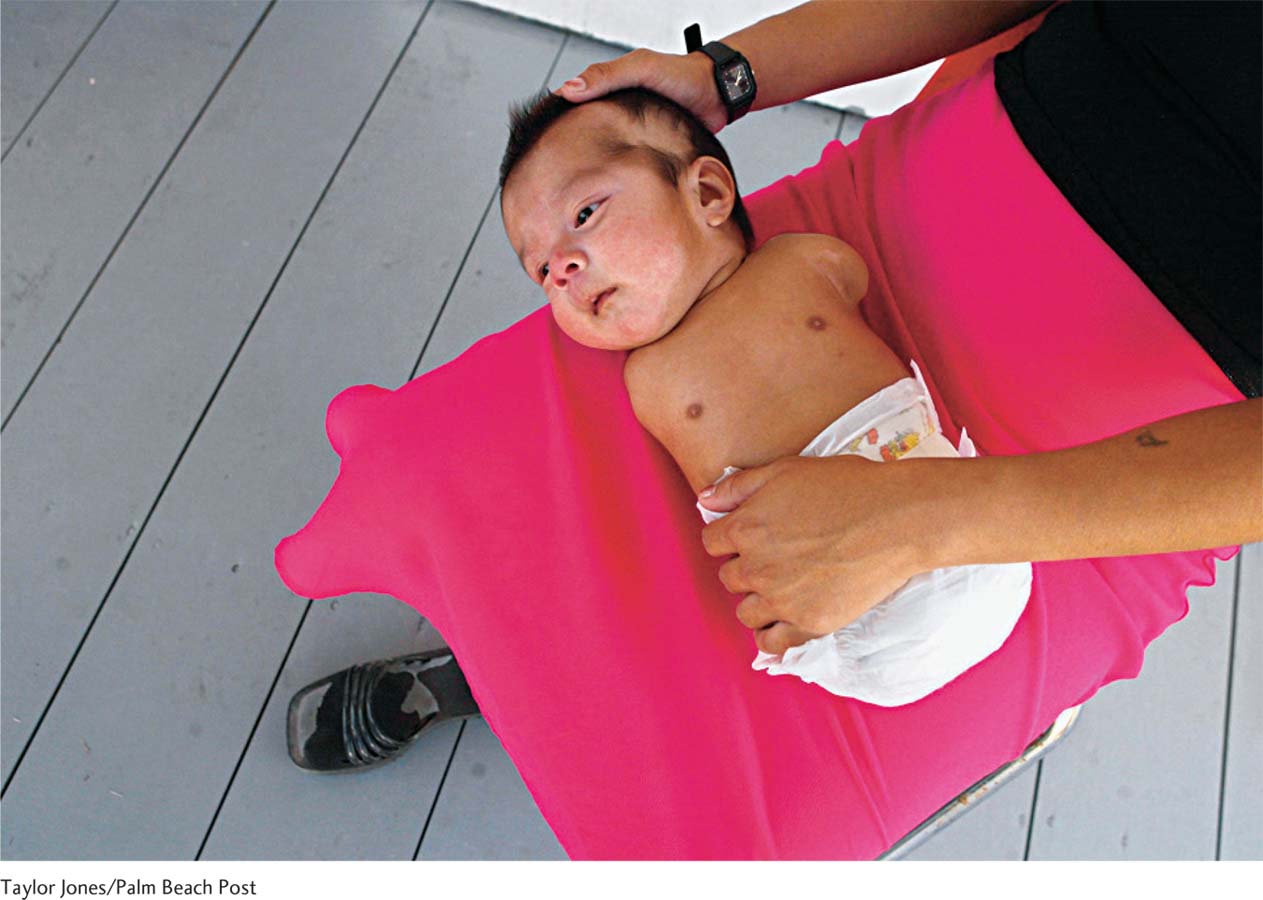
The universal fears about the growing baby are expressed in cultural prohibitions: “Don’t use scissors or your baby will have cut lips” (a cleft palate) Afghanistan; “Avoid looking at monkeys [Indonesia] or gossiping [China] or your baby will be deformed.”
If you think these practices are strange, consider the standard mid-
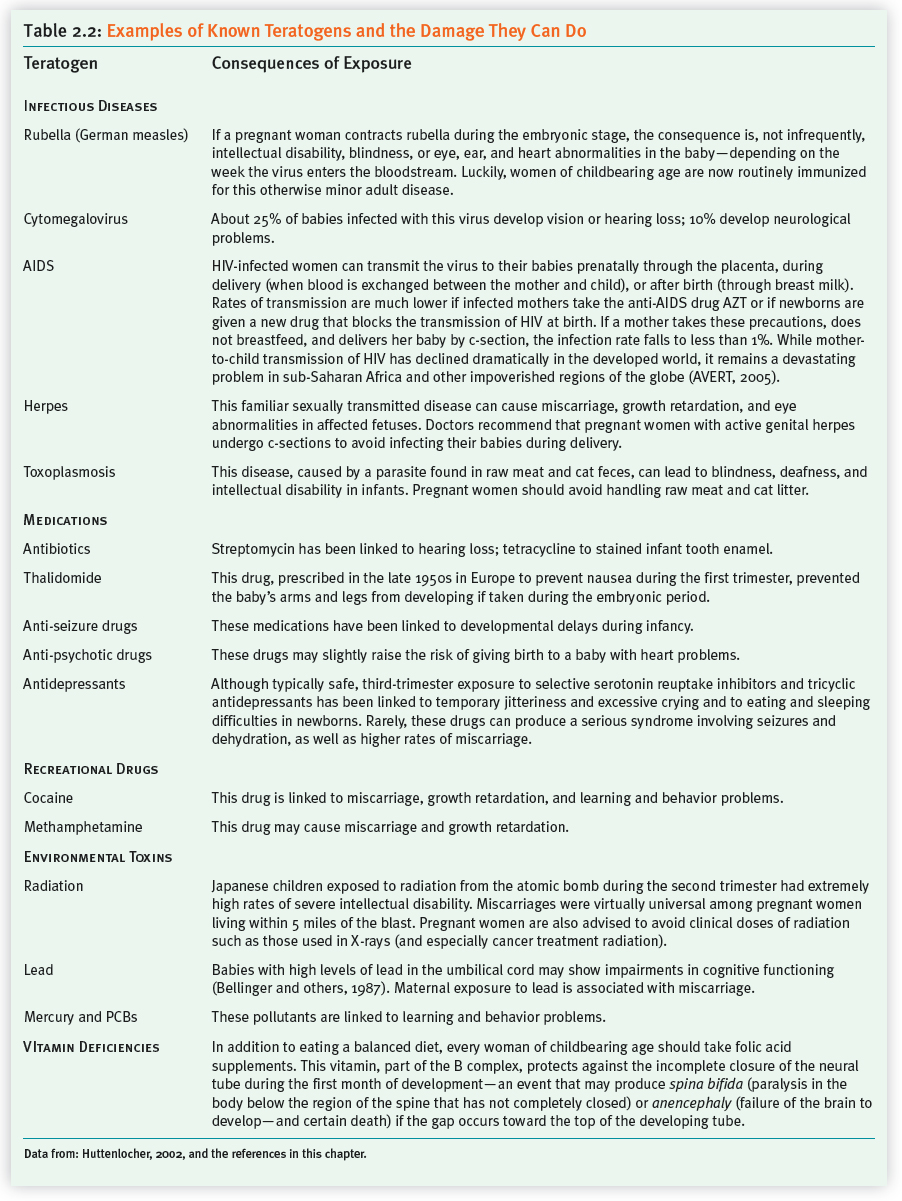
A teratogen (from the Greek word teras, “monster,” and gen, “creating”) is the name for any substance that crosses the placenta to harm the fetus. A teratogen may be an infectious disease; a medication; a recreational drug; environmental hazards, such as radiation or pollution; or as you will see later, the hormones produced by a pregnant woman who is under extreme stress. Table 2.2 describes potential teratogens in various categories.
47
Basic Teratogenic Principles
Teratogens typically exert their damage during the sensitive period—the timeframe when a particular organ or system is coming “on line.” For example, the infectious disease called rubella (German measles) often damaged a baby’s heart or ears, depending on the week during the first trimester when a mother contracted the disease. The sedative Thalidomide, prescribed in Europe during the late 1950s to prevent morning sickness, impaired limb formation, depending on which day after fertilization the drug was imbibed. In general, with regard to teratogens, the following principles apply:
Teratogens are most likely to cause major structural damage during the embryonic stage. Before implantation, teratogens have an all-
or- nothing impact. They either inhibit implantation and cause death, or they leave the not- yet- attached blastocyst unscathed. It is during organ formation (after implantation through week 8) that major body structures are most likely to be affected. This is why— unless expectant mothers have a chronic disease that demands continuing— physicians advise forgoing any medications during the first trimester (American Academy of Pediatrics [AAP], Committee on Drugs, 2000). Teratogens can affect the developing brain throughout pregnancy. As you saw earlier, because the brain is forming during the second and third trimesters, the potential for neurological damage extends for all nine months. Typically, during the second and third trimesters, exposure to teratogens increases the risk of developmental disorders. This term refers to any condition that compromises normal development—
from delays in reaching basic milestones, such as walking or talking, to learning problems and hyperactivity. 48
Teratogens have a threshold level above which damage occurs. For instance, women who drink more than four cups of coffee a day throughout pregnancy have a slightly higher risk of miscarriage; but having an occasional Diet Coke is fine (Gilbert-
Barness, 2000). Teratogens exert their damage unpredictably, depending on fetal and maternal vulnerabilities. Still, mothers-
to- be metabolize toxins differently, and babies differ genetically in susceptibility. So the damaging effects of a particular teratogen can vary. On the plus side, you may know a child in your local school’s gifted program whose mother drank heavily during pregnancy. On the negative side, we do not know where the teratogenic threshold lies in any particular case. Therefore, during pregnancy, it’s best to err on the side of caution.
Although the damaging impact of a teratogen may show up during infancy, it can also manifest itself years later. An unfortunate example of this teratogenic time bomb took place in my own life. My mother was given a drug called diethylstilbestrol (DES) while she was pregnant with me. (DES was prescribed routinely in the 1950s and 1960s to prevent miscarriage.) During my early twenties, I developed cancerous cells in my cervix—
The Teratogenic Impact of Medicines and Recreational Drugs
The fact that some medications are teratogenic presents dilemmas for women. Do you stop taking your anti-
As these comments illustrate, with medications and pregnancy, it can be a balancing act. Sometimes there are no perfect choices.
With recreational drugs, the choice is clear. Each substance is potentially teratogenic. So it’s best to just say no!
Because tobacco and alcohol are woven into the fabric of daily life, let’s now focus on these widely used teratogens. What can happen to the baby when pregnant women smoke and drink?
SMOKING Each time she reads the information on a cigarette pack, a pregnant woman gets a reminder that she may be doing her baby harm. Still, with polls showing that anywhere from 1 in 12 people (in the Netherlands) and 1 in 3 pregnant women (in Spain) smoke, tobacco use during this time is far from rare (De Wilde and others, 2013). Because this practice is such a “no, no,” these surveys almost certainly underestimate the fraction of smoking mothers-
The main danger with smoking is giving birth to a smaller-
49
The good news is that the many U.S. pregnant smokers who take the difficult step of quitting for the health of their babies (see Chisolm, Cheng, & Terplan, 2014) feel more efficacious and less depressed (De Wilde and others, 2013). The bad news is that women still get ammunition—
ALCOHOL As you saw earlier, it used to be standard to encourage pregnant women to have a nightcap to relieve stress. In Italy, drinking red wine during pregnancy was supposed to produce a healthy, rosy-
Women who binge-

Faced with these warnings, New Zealand researchers found about half of women in a national poll reported stopping drinking after learning they were pregnant (Parackal Parackal, & Harraway, 2013). Ironically, however, trying to conceive has no influence on alcohol use (Terplan, Cheng, & Chisolm, 2014). Pregnant women who drink regularly tend to be anxious or depressed (Beijers and others, 2014). Surprisingly, however, one study in the Netherlands showed well-
This unexpected finding may reflect cultural norms. Every U.S. public health organization recommends no alcohol during pregnancy. In Europe, having a cocktail or glass of wine is an expected practice during meals. This may explain why European physicians disagree with their U.S. counterparts: “One drink per day can’t possibly do the fetus harm” (Paul, 2010; Royal College of Obstetricians and Gynaecologists [RCOG], 1999).
Measurement Issues
Why is there any debate about a safe amount of alcohol to drink? For answers, imagine the challenges you would face as a researcher exploring the impact of tobacco or alcohol on the developing child: The need to ask thousands of pregnant women to estimate how often they indulged in these “unacceptable” behaviors and then track the children for decades, looking for problems that might appear as late as adult life. Plus, because your study is correlational, the difficulties you find might be due to other confounding causes. Pregnant women who drink are more likely to smoke (Mallard, Connor, & Houghton, 2013). As I’ve implied, these people may be generally stressed out. Could you isolate the child’s symptoms to just tobacco or alcohol? Wouldn’t simply feeling overly anxious damage the developing child?
50
Hot in Developmental Science: What Is the Impact of Prenatal Stress?
I introduced this chapter by emphasizing that anxiety is normal during pregnancy. Will my baby be all right? I discussed how throughout history people intuitively believed that stress could harm the fetus, so societies went to heroic lengths to keep mothers calm. What does the research suggest about the impact of pregnancy stress?
One concern is that excessive anxiety may cause premature labor, causing women to miscarry or have an unhealthy infant (Guardino & Schetter, 2014). High levels of the stress hormone cortisol, as it turns out, are transmitted to the fetus via the amniotic fluid, making babies irritable during the first months of life (Baibazarova and others, 2013). Now—
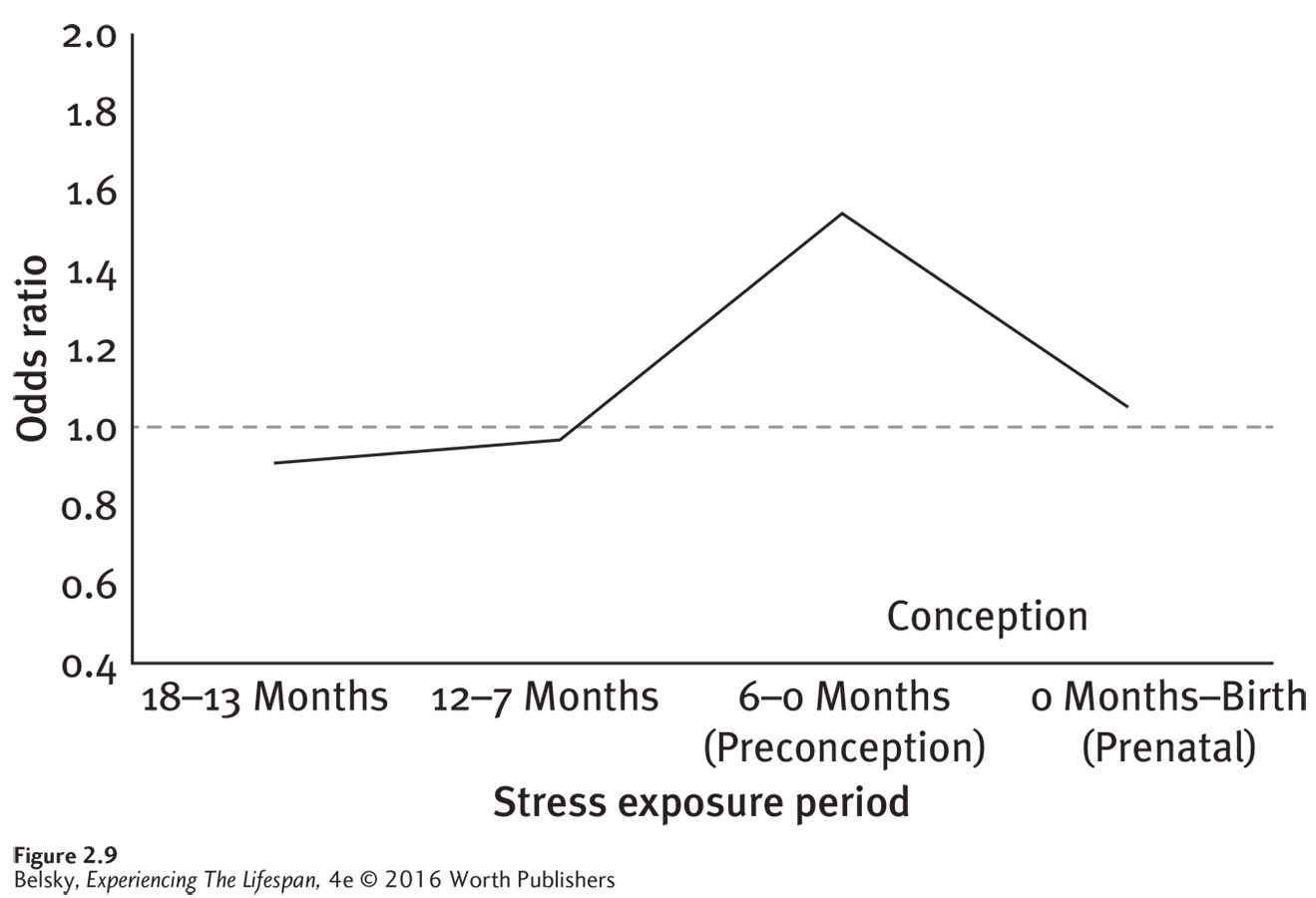
The intensity, quality, and timing of the stress may matter. Does the person have an overload of problems, few social supports, or is she experiencing a difficult (perhaps unwanted) pregnancy? Anxieties about fetal health, not unexpectedly, are more common in older moms (Bayrampour and others, 2013). One study showed excessive stress in the latter part of pregnancy increased the chance of premature labor (Cole-
Lewis and others, 2014). Then, ironically— as if women didn’t have enough to worry about— notice from Figure 2.9, that other researchers discovered traumatic events prior to getting pregnant compromised the baby’s health (Class and others, 2013)! The person’s personality and coping style matters most. In thoughtfully reviewing these confusing findings, developmentalists concluded that the critical variable relates to the way women handle stress (Guardino & Schetter, 2014). Does the person behave proactively, taking constructive steps to confront problems, or bury her difficulties by using avoidant strategies? Denying distress, or passively breaking down, and, of course, resorting to binge drinking or smoking to cope, raises the risk of giving birth early and having a frail child.
But suppose the trauma is so overwhelming that it’s impossible to constructively cope. Imagine that while a woman is pregnant a disaster occurs—
Is Pregnancy a Programmer of Old Age?
The answer may be yes. For instance, during World War II, in 1944, the Germans cut off the food supply to Holland, putting that nation in a semi-
51
Why might deprivation in the womb be linked to premature, age-

Is obesity (and adult chronic disease) caused just by personal lifestyle choices or partly promoted by a poor body-
Freud revolutionized the twentieth century by arguing that childhood experiences shape personality. Will twenty-
Fetal programming research is action oriented. Ideally, we can take steps before birth to influence a child’s fate. With these next conditions, the problems are often more serious. They are frequently diagnosed at birth. This is because the child’s condition is “genetic.” It was sealed at conception with the union of an egg and sperm.
Threats from Within: Chromosomal and Genetic Disorders
When a birth defect is classified as “genetic,” there are two main causes. The child might have an unusual number of chromosomes, or a faulty gene (or set of genes) might be the problem.
Chromosomal Problems
As we know, the normal human chromosomal complement is 46. However, sometimes a baby with a missing or extra chromosome is conceived. The vast majority of these fertilizations end in first-
Still, babies can be born with an abnormal number of sex chromosomes (such as an extra X or two, an extra Y, or a single X) and survive. In this case, although the symptoms vary, the result is often learning impairments and sometimes infertility.
Survival is also possible when a child is born with an extra chromosome on a specific other pair. The most common example—
Down syndrome typically occurs because a cell-
This extra chromosome produces familiar physical features: a flat facial profile, an upward slant to the eyes, a stocky appearance, and an enlarged tongue. Babies born with Down syndrome are at high risk for having heart defects and childhood leukemia. Here, too, there is a lifespan time-
A century ago, Down syndrome children rarely lived to adulthood. They were shunted to institutions to live severely shortened lives. In the United States today, due to medical advances, these babies have an average life expectancy of 60 years (NDSS, retrieved 2014). Ironically, this longevity gain can be a double-
52

This is not to say that every Down syndrome baby is dependent on a caregiver’s help. These children can sometimes learn to read and write. They can live independently, hold down jobs, marry and have children, construct fulfilling lives. Do you know a child with Down syndrome like the toddler in this photo who is the light of her loving family and friends’ lives?
Although women of any age can give birth to Down syndrome babies, the risk rises exponentially among older mothers. Over age 40, the chance of having a Down syndrome birth is 1 in 100; over age 45, it is 1 in 30 (NDSS, n.d.). The reason is that, with more time “in storage,” older ova are more apt to develop chromosomal faults.
Down syndrome is typically caused by a spontaneous genetic mistake. Now let’s look at a different category of genetic disorders—
Genetic Disorders
Most illnesses—
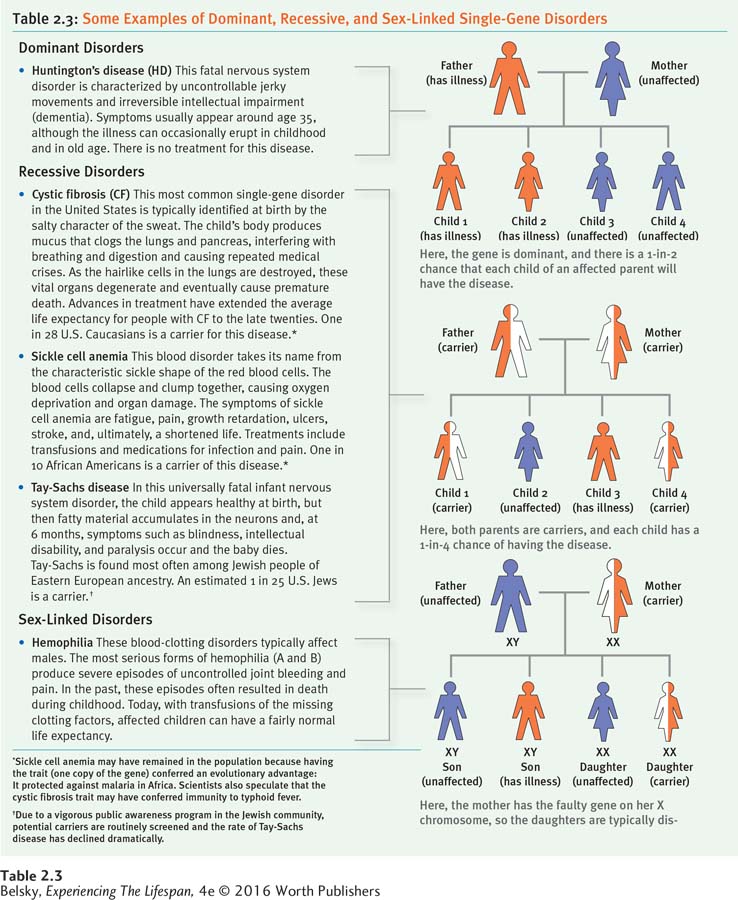
Single-
Dominant disorders are in the first category. In this case, if one parent harbors the problem gene (and so has the illness), each child the couple gives birth to has a fifty-
Recessive disorders are in the second category. Unless a person gets two copies of the gene, one from the father and one from the mother, that child is disease free. In this case, the odds of a baby born to two carriers—
The mode of transmission for sex-
53
Because their single X leaves them vulnerable, sex-
Table 2.3 visually decodes these modes of inheritance and describes a few of the best-
54
With the other illnesses in the table—
The good news, as the table shows, is that the prognoses for some routinely fatal childhood single-
HOW DO WE KNOW . . .
about the gene for Huntington’s disease?
Nancy Wexler and her sister got the devastating news from their physician father, Milton: “Your mother has Huntington’s disease. She will die of dementia in a horrible way. As a dominant single gene disorder, your chance of getting ill is fifty-
A breakthrough came in 1979, when Nancy learned that the world’s largest group of people with Huntington’s lived in a small, inbred community in Venezuela—

Having this diagnostic marker is the first step to eventually finding a cure. So far the cure is elusive, but the hunt continues. Nancy still serves as the head of the foundation, vigorously agitating for research on the illness that killed her mother. She works as a professor in Columbia University’s Neurology and Psychiatry Department. But every year, she comes back to the village in Venezuela to counsel and just visit with her families—
55
In sum, the answer to the question “Can single-
Our most dramatic progress, however, lies in genetic testing. Through a simple blood test, people can find out whether they carry the gene for these (and other) illnesses.
These diagnostic breakthroughs bring up difficult issues. Would you really want to know whether you have the gene for Huntington’s disease? The inspiring story of Nancy Wexler, the psychologist who helped discover the Huntington’s gene and whose mother died of the disease, is instructive here (see the How Do We Know box). While Nancy will not say whether she has been tested, her sister Alice refused to be screened because she felt not knowing was better emotionally than the anguish of living with a positive result.
Interventions
The advantages of genetic testing are clearer when the issue relates to having a child. Let’s imagine for instance that you and your spouse know you are carriers of the cystic fibrosis gene. If you are contemplating having children, what should you do?
Sorting Out the Options: Genetic Counseling
Your first step would be to consult a genetic counselor, a professional skilled in both genetics and counseling, to help you think through your choices. In addition to laying out the odds of having an affected child, genetic counselors describe advances in treatment. For example, they inform couples who are carriers for cystic fibrosis about biological strategies on the horizon, such as gene therapy. They also highlight the interpersonal and economic costs of having a child with this disease. But they are trained never to offer advice. Their goal is to permit couples to make a mutual decision on their own.
Now, suppose that armed with this information, you and your partner go ahead and conceive. Let’s scan the major tests that are available to every woman carrying a child.
Tools of Discovery: Prenatal Tests
Blood tests performed during the first trimester can detect (with reasonable accuracy) various chromosomal conditions, such as Down syndrome. Brain scans (MRIs) offer a vivid prenatal window on the developing brain (Jokhi & Whitby, 2011). The standard fetal diagnostic test has been a staple for over 40 years: the ultrasound.
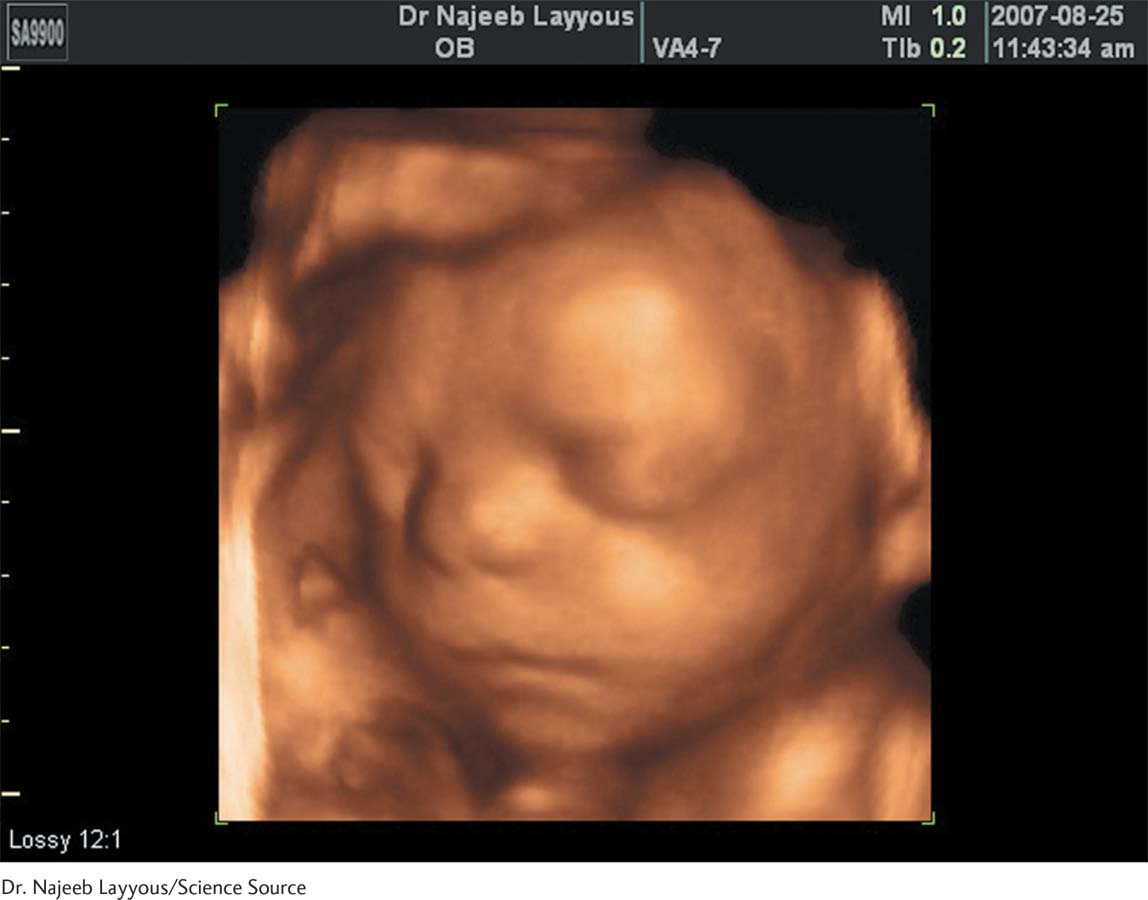
Ultrasounds, which now provide a clear image of fetus (see the accompanying photo), are used to date the pregnancy and assess in utero growth, in addition to revealing physical abnormalities. By making the baby visually real, ultrasound visits have become emotional landmarks on the pregnancy journey itself (Paul, 2010). Imagine the thrill of getting this vivid photo of your baby months before she is born!
Pregnant women embrace ultrasound technology and noninvasive genetic tests (Verweij and others, 2013). They tend to be more wary of the next procedures, because those require entering the womb.
During the first trimester, chorionic villus sampling (CVS) can diagnose a variety of chromosomal and genetic conditions. A physician inserts a catheter into the woman’s abdomen or vagina and withdraws a piece of the developing placenta for analysis. The advantage of CVS, is knowing early on about problems; however, this test can be slightly dangerous, as it carries a risk of miscarriage and limb impairments (Karni, Leshno, & Rapaport, 2014).
56
During the second trimester, a safer test, called amniocentesis, can determine the fetus’s fate. The doctor inserts a syringe into the woman’s uterus and extracts a sample of amniotic fluid. The cells can reveal a host of genetic and chromosomal conditions, as well as the fetus’s sex.
Amniocentesis is planned for a gestational age (typically week 14) when there is enough fluid to safely siphon out and time to decide whether or not to carry the baby to term. However, it, too, carries a small chance of infection and miscarriage, depending on the skill of the doctor performing the test (Karni, Lescho & Rapaport, 2014). Moreover by the time the results of the “amnio” arrive, quickening may have occurred. The woman must endure the trauma of labor should she decide to terminate the pregnancy at this late stage.
Because their risk of having a child with chromosomal disorders is higher, many doctors suggest that patients over age 35 have these procedures. But, not unexpectedly, more women in their forties agree to tested; and, because it is safer, more people undergo amniocentesis than CVS (Godino and others, 2013).
When these couples receive a diagnosis of serious chromosomal problems, most do terminate the pregnancy—
The summary timelines above show these procedures and charts the landmark events of prenatal development and pregnancy. I cannot emphasize strongly enough that giving birth to a baby with serious birth defects is rare. That is not true of the topic I turn to now—
Infertility and New Reproductive Technologies
I’ll never forget the comment my sister made . . . when I was about 13. I said, “I’m a woman because I have my period” . . . . And she said, “You are not a woman until you have a baby” (from a woman who after years of infertility adopted a child).
(quoted in Loftus & Androit, 2012, p. 23)
According to Psalm 127:3, “Children are a heritage unto the Lord and the fruit of his womb is His Reward.” So why didn’t I get this gift? I asked myself over and over if I was being punished.
(quoted in Ferland and Caron, 2013, p. 183)
These quotations have an ageless quality. Since biblical times, humanity has equated womanhood with bearing a child. The message that “being barren” is a terrible, female fate is an underlying message beginning in Genesis. When his beloved wife Sarah couldn’t get pregnant, the Biblical patriarch Abraham felt compelled to “procreate” with a substitute wife.
57
Infertility—the inability to conceive a child after a year of unprotected intercourse—
While infertility can affect women (and men) of every age, just as with miscarriage and Down syndrome—
Infertility puts stress on both partners. Still, as the quotes at the beginning of this section suggest, this life trauma is apt to hit women hardest (Teskereci & Oncel, 2013). Although they are more immune from feelings of having personally failed (Herrera, 2013), males have pressures to prove their manhood by fathering a child. In one Danish questionnaire study, almost 1 in 3 patients at a male fertility clinic confessed that their condition affected their sense of masculinity and self-
The impact varies in intensity, depending on one’s culture. In places like Iran, where not being able to bear a child is sometimes an accepted reason for divorce (more about this in Chapter 11), infertility can leave a woman shunned by family and friends (Behboodi-
And, when you are in these situations, do you discuss your situation, or clam up? Revealing your problem to parents—
Does telling people help? If, and only if, you have a caring, social-
58
When I told him (my second husband) when we were dating that I could not have children, he said, “If god wanted me to have kids, he would have made me fall in love with a woman who could have them.”
(quoted in Ferland & Caron, 2013, p. 186)
Just as with the Biblical patriarch Abraham, whose decision to stay with Sarah is an ageless model for marital love, infertility can offer a chance to demonstrate a person’s loving commitment to a mate.
Today, communicating collaboratively around fertility issues is essential, as science offers couples so many options to help fulfill the quest to have a (partly) biological child.
INTERVENTIONS: Exploring ART
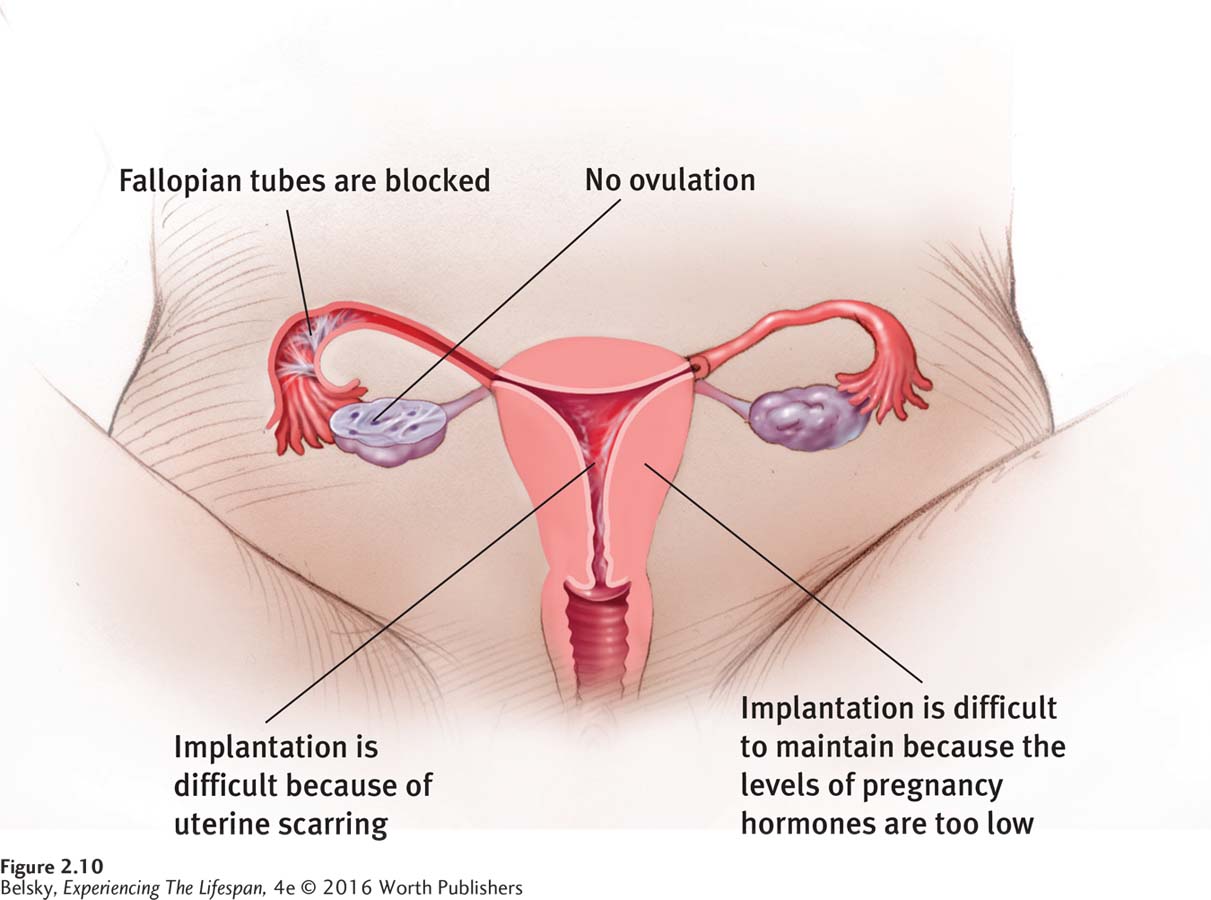
For females, there are treatments to attack every problem on the reproductive chain (see the illustration in Figure 2.10)—from fertility drugs to stimulate ovulation, to hormonal supplements to foster implantation; from surgery to help clean out the uterus and the fallopian tubes, to artificial insemination (inserting the sperm into the woman’s uterus through a syringe). Males may take medications or undergo surgery to increase the quality and motility of the sperm. Then there is that ultimate approach: assisted reproductive technology (ART).
Assisted reproductive technology refers to any strategy in which the egg is fertilized outside the womb. The most widely used ART procedure is in vitro fertilization (IVF). After the woman is given fertility drugs (which stimulate multiple ovulations), her eggs are harvested and put in a laboratory dish, along with the partner’s sperm, to be fertilized. A few days later, the fertilized eggs are inserted into the uterus. Then, the couple anxiously waits to find out if the cells have implanted in the uterine wall.
In vitro fertilization, initially developed to bypass blocked fallopian tubes, has spawned amazing variations. A sperm may be injected directly into the ovum if it cannot penetrate the surface on its own. The woman may use a donor egg—
Imagine the emotions that can arise when another person is carrying your baby, or if the child you are carrying has another woman’s (or man’s) genes. And, consider the expense of these added “pregnancy players.” As the cost of soliciting a donor egg can be as high as $30,000, and fees to the donor vary from $5,000 to $15,000, an ART investment can top $40,000—
59
Now, imagine enduring the invasive techniques used to harvest and insert the eggs, and managing your monthly anguish if a pregnancy doesn’t occur. According to 2012 U.S. data, the odds of a woman under age 35 getting pregnant after a round of in vitro treatments was less than fifty/fifty. Over age 42, success rates per cycle slid down to less than 1 in 10 (Society for Assisted Reproductive Technologies, retrieved 2014).
Critics emphasize the headaches (and heartaches) involved in ART; the pain, expense, and the chance of miscarrying if many eggs take (often to counter this risk, doctors engage in a procedure gently named “fetal reduction”); the virtual certainty of having fragile, small babies when several conceptions come to term (Gentile, 2014; Centers for Disease Control and Prevention (CDC), retrieved 2014); or the issues attached to third-
These complaints ignore the gift ART provides. This landmark technology has given thousands of infertile couples their only chance to have a biological child: “I could never have accomplished all of this myself,” gushed one grateful Taiwanese woman. Another said: “I no longer felt pitiful. . . . My child represents the continuation of my life” (quoted in Lin, Tsai, and Lai, 2013, p. 194).
Tying It All Together
Question 2.12
Teratogen A caused limb malformations. Teratogen B caused developmental disorders. Teratogen A wreaked its damage during the___________ stage of prenatal development and was taken during the____________ trimester of pregnancy, while teratogen B probably did its damage during the ___________stage and was taken during the __________ trimester.
Teratogen A most likely caused damage during the embryonic stage of development and was taken during the first trimester of pregnancy. Teratogen B probably did its damage during the fetal stage and was taken during the second or third trimesters.
Question 2.13
Seto and Brandon’s mothers contracted rubella (German measles) during different weeks in their first trimester of pregnancy. Seto has heart problems; Brandon has hearing problems. Which teratogenic principle is illustrated here?
Teratogens exert damage during the sensitive period for the development of a particular organ.
Question 2.14
Monique is planning to become pregnant and asks her physician if it will be okay for her to have a glass of wine with dinner each night. What would her doctor answer if Monique lived in the United States? What might the doctor say if Monique lived in France?
A doctor in the United States would advocate no alcohol, while a physician in France might say a glass of wine is fine.
Question 2.15
Imagine that in 2016, a tornado hits Nashville, Tennessee. Based on the fetal programming research, which two predictions might you make about babies who were in-
They might be at higher risk of being born small.
They might be at higher risk of developing premature heart disease.
They might be at higher risk of being very thin throughout life.
a & b. They might be at higher risk of being born small and of developing premature heart disease.
Question 2.16
Latasha gives birth to a child with Down syndrome, while Jennifer gives birth to a child with cystic fibrosis. Which woman should be more worried about having another child with that condition, and why?
Jennifer. Down syndrome is typically caused by an unlikely, random event. With cystic fibrosis, that single-
Question 2.17
To a friend who is thinking of choosing between chorionic villus sampling (CVS) and amniocentesis, mention the advantages and disadvantages of each procedure.
Tell your friend that the plus of chorionic villus sampling is finding out a child's genetic fate in the first trimester. However, this procedure is more dangerous, carrying a slight risk of limb malformations and, possibly, miscarriage. Amniocentesis is much safer and can show a fuller complement of genetic disorders but must be performed in the second trimester-
Question 2.18
Jennifer and Brad are considering ART, after years of unsuccessful fertility treatments. First describe some pros and cons of this procedure. According to the text, what force is most critical in determining how well Jennifer has been coping with her troubles getting pregnant?
Cons: ART can be expensive, demands effort and time, has unpleasant physical symptoms, and the chance of actually getting pregnant per cycle is small—
60
Now let’s look at what happens when these wished-