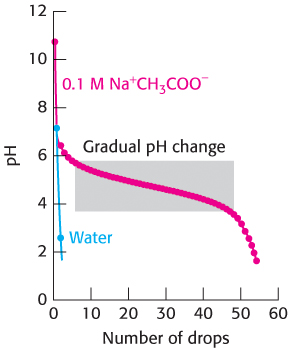
FIGURE 1.17 Buffer action. The addition of a strong acid, 1 M HCl, to pure water results in an immediate drop in pH to near 2. In contrast, the addition of the acid to a 0.1 M sodium acetate (Na− CH3COO−) solution results in a much more gradual change in pH until the pH drops below 3.5.
[Leave] [Close]