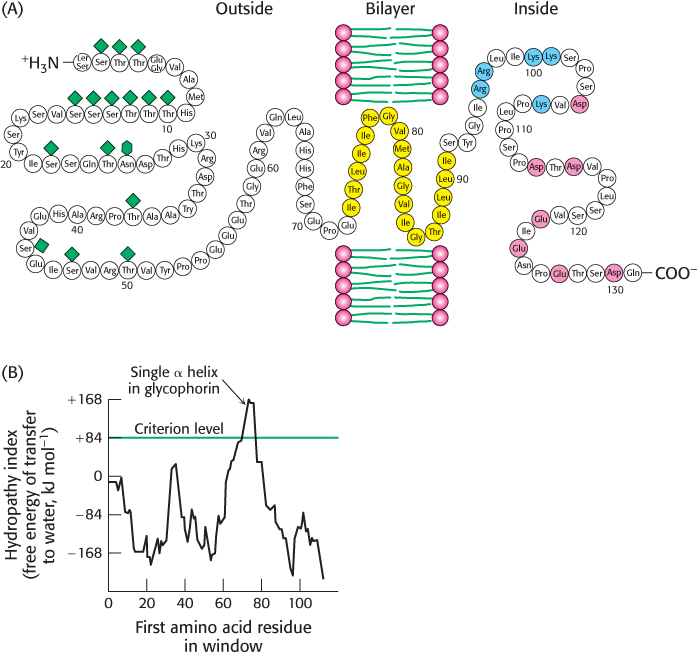
FIGURE 12.26 Locating the membrane- spanning helix of glycophorin. (A) Amino acid sequence and transmembrane disposition of glycophorin A from the red- blood- cell membrane. Fifteen O-linked carbohydrate units are shown as diamond shapes, and an N-linked unit is shown as a lozenge shape. The hydrophobic residues (yellow) buried in the bilayer form a transmembrane α helix. The carboxyl- terminal part of the molecule, located on the cytoplasmic side of the membrane, is rich in negatively charged (red) and positively charged (blue) residues. (B) Hydropathy plot for glycophorin. The free energy for transferring a helix of 20 residues from the membrane to water is plotted as a function of the position of the first residue of the helix in the sequence of the protein. Peaks of greater than +84 kJ mol−1 (+20 kcal mol−1) in hydropathy plots are indicative of potential transmembrane helices.
[(A) Information from Dr. Vincent Marchesi; (B) data from D. M. Engelman, T. A. Steitz, and A. Goldman, Annu. Rev. Biophys. Biophys. Chem. 15:321– 353, 1986. Copyright © 1986 by Annual Reviews, Inc. All rights reserved.]
[Leave] [Close]