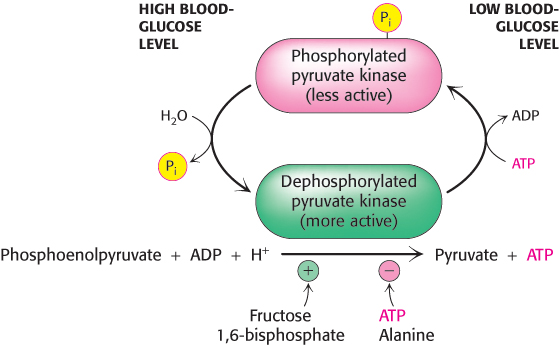
FIGURE 16.21 Control of the catalytic activity of pyruvate kinase. Pyruvate kinase is regulated by allosteric effectors and covalent modification. Fructose 1,6- bisphosphate allosterically stimulates the enzyme, while ATP and alanine are allosteric inhibitors. Glucagon, secreted in response to low blood glucose, promotes phosphorylation and inhibition of the enzyme. When blood glucose levels are adequate, the enzyme is dephosphorylated and activated.
[Leave] [Close]