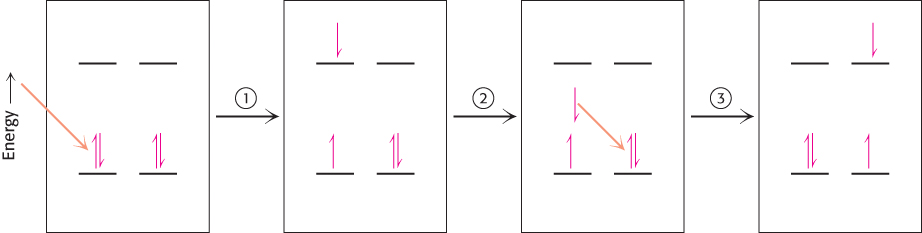
Resonance energy transfer. (1) An electron can accept energy from electromagnetic radiation of appropriate wavelength and jump to a higher energy state. (2) When the excited electron falls back to its lower energy state, the absorbed energy is released. (3) The released energy can be absorbed by an electron in a nearby molecule, and this electron jumps to a high energy state.