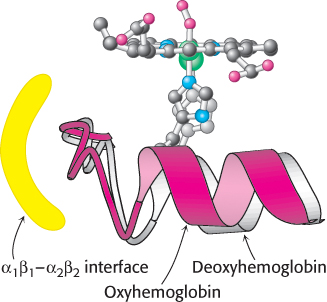
FIGURE 7.15 Conformational changes in hemoglobin. The movement of the iron ion on oxygenation brings the iron- associated histidine residue toward the porphyrin ring. The associated movement of the histidine- containing α helix alters the interface between the αβ dimers, instigating other structural changes. For comparison, the deoxyhemoglobin structure is shown in gray behind the oxyhemoglobin structure in red.
[Leave] [Close]