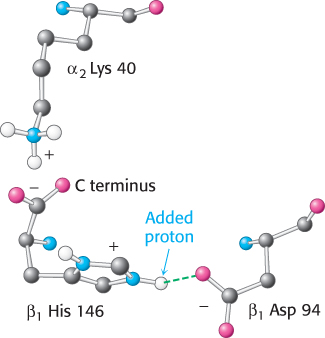
FIGURE 7.20 Chemical basis of the Bohr effect. In deoxyhemoglobin, three amino acid residues form two salt bridges that stabilize the T quaternary structure. The formation of one of the salt bridges depends on the presence of an added proton on histidine β146. The proximity of the negative charge on aspartate β94 in deoxyhemoglobin favors protonation of this histidine. Notice that the salt bridge between histidine β146 and aspartate β94 is stabilized by a hydrogen bond (green dashed line).
[Leave] [Close]