PROBLEMS
PROBLEMS
Question 7.1
Screening the biosphere. The first protein structure to have its structure determined was myoglobin from sperm whale. Propose an explanation for the observation that sperm whale muscle is a rich source of this protein.
Question 7.2
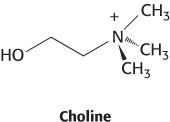
Hemoglobin content. The average volume of a red blood cell is 87 μm3. The mean concentration of hemoglobin in red cells is 0.34 g ml−1.
213
What is the weight of the hemoglobin contained in an average red cell?
How many hemoglobin molecules are there in an average red cell? Assume that the molecular weight of the human hemoglobin tetramer is 65 kDa.
Could the hemoglobin concentration in red cells be much higher than the observed value? (Hint: Suppose that a red cell contained a crystalline array of hemoglobin molecules in a cubic lattice with 65 Å sides.)
Question 7.3
Iron content. How much iron is there in the hemoglobin of a 70-
Question 7.4
Oxygenating myoglobin. The myoglobin content of some human muscles is about 8 g kg−1. In sperm whale, the myoglobin content of muscle is about 80 g kg−1.
How much O2 is bound to myoglobin in human muscle and in sperm whale muscle? Assume that the myoglobin is saturated with O2, and that the molecular weights of human and sperm whale myoglobin are the same.
The amount of oxygen dissolved in tissue water (in equilibrium with venous blood) at 37°C is about 3.5 × 10−5 M. What is the ratio of oxygen bound to myoglobin to that directly dissolved in the water of sperm whale muscle?
Question 7.5
Tuning proton affinity. The pKa of an acid depends partly on its environment. Predict the effect of each of the following environmental changes on the pKa of a glutamic acid side chain.
A lysine side chain is brought into proximity.
The terminal carboxyl group of the protein is brought into proximity.
The glutamic acid side chain is shifted from the outside of the protein to a nonpolar site inside.
Question 7.6
Saving grace. Hemoglobin A inhibits the formation of the long fibers of hemoglobin S and the subsequent sickling of the red cell on deoxygenation. Why does hemoglobin A have this effect?
Question 7.7
Carrying a load. Suppose that you are climbing a high mountain and the oxygen partial pressure in the air is reduced to 75 torr. Estimate the percentage of the oxygen-
Question 7.8
Bohr for me, not for thee. Does myoglobin exhibit a Bohr effect? Why or why not?
Question 7.9
High-
Question 7.10
Blood doping. Endurance athletes sometimes try an illegal method of blood doping called autologous transfusion. Some blood from the athlete is removed well before competition, and then transfused back into the athlete just before competition.
Why might blood transfusion benefit the athlete?
With time, stored red blood cells become depleted in 2,3-
BPG. What might be the consequences of using such blood for a blood transfusion?
Question 7.11
I’ll take the lobster. Arthropods such as lobsters have oxygen carriers quite different from hemoglobin. The oxygen-
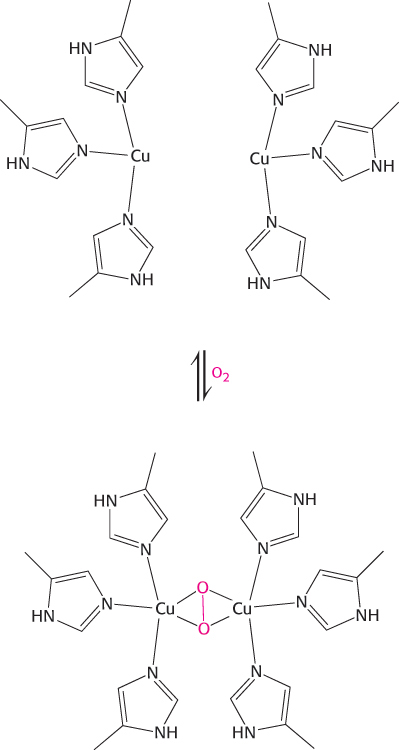
Question 7.12
A disconnect. With the use of site-
214
Question 7.13
Successful substitution. Blood cells from some birds do not contain 2,3-
Question 7.14
Theoretical curves. (a) Using the Hill equation, plot an oxygen-
Question 7.15
Parasitic effect. When P. falciparum lives inside red blood cells, the metabolism of the parasite tends to release acid. What effect is the presence of acid likely to have on the oxygen-
Data Interpretation Problems
Question 7.16
Primitive oxygen binding. Lampreys are primitive organisms whose ancestors diverged from the ancestors of fish and mammals approximately 400 million years ago. Lamprey blood contains a hemoglobin related to mammalian hemoglobin. However, lamprey hemoglobin is monomeric in the oxygenated state. Oxygen-
|
pO2 |
Y |
pO2 |
Y |
pO2 |
Y |
|---|---|---|---|---|---|
|
0.1 |
.0060 |
2.0 |
.112 |
50.0 |
.889 |
|
0.2 |
.0124 |
3.0 |
.170 |
60.0 |
.905 |
|
0.3 |
.0190 |
4.0 |
.227 |
70.0 |
.917 |
|
0.4 |
.0245 |
5.0 |
.283 |
80.0 |
.927 |
|
0.5 |
.0307 |
7.5 |
.420 |
90.0 |
.935 |
|
0.6 |
.0380 |
10.0 |
.500 |
100 |
.941 |
|
0.7 |
.0430 |
15.0 |
.640 |
150 |
.960 |
|
0.8 |
.0481 |
20.0 |
.721 |
200 |
.970 |
|
0.9 |
.0530 |
30.0 |
.812 |
|
|
|
1.0 |
.0591 |
40.0 |
.865 |
|
|
Plot these data to produce an oxygen-
binding curve. At what oxygen partial pressure is this hemoglobin half- saturated? On the basis of the appearance of this curve, does oxygen binding seem to be cooperative? Construct a Hill plot using these data. Does the Hill plot show any evidence for cooperativity? What is the Hill coefficient?
Further studies revealed that lamprey hemoglobin forms oligomers, primarily dimers, in the deoxygenated state. Propose a model to explain any observed cooperativity in oxygen binding by lamprey hemoglobin.
Question 7.17
Leaning to the left or to the right. The illustration below shows several oxygen-
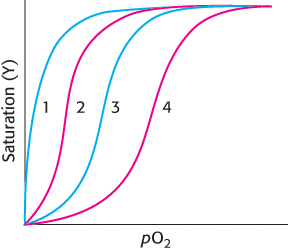
Decrease in CO2
Increase in 2,3-
BPG Increase in pH
Loss of quaternary structure
Chapter Integration Problems
Question 7.18
Location is everything. 2,3-
Question 7.19
A therapeutic option. Hydroxyurea has been shown to increase the expression of fetal hemoglobin in adult red blood cells, by a mechanism that remains unclear. Explain why hydroxyurea can be a useful therapy for patients with sickle-