SUMMARY
SUMMARY
8.1 Enzymes Are Powerful and Highly Specific Catalysts
Most catalysts in biological systems are enzymes, and nearly all enzymes are proteins. Enzymes are highly specific and have great catalytic power. They can enhance reaction rates by factors of 106 or more. Many enzymes require cofactors for activity. Such cofactors can be metal ions or small, vitamin-
8.2 Gibbs Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes
Free energy (G) is the most valuable thermodynamic function for understanding the energetics of catalysis. A reaction can take place spontaneously only if the change in free energy (ΔG) is negative. The free-
8.3 Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State
Enzymes serve as catalysts by decreasing the free energy of activation of chemical reactions. Enzymes accelerate reactions by providing a reaction pathway in which the transition state (the highest-
The first step in catalysis is the formation of an enzyme–
8.4 The Michaelis–
The kinetic properties of many enzymes are described by the Michaelis–
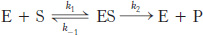
244
The rate of formation of product V0 is given by the Michaelis–
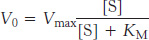
in which Vmax is the reaction rate when the enzyme is fully saturated with substrate and KM, the Michaelis constant, is the substrate concentration at which the reaction rate is half maximal. The maximal rate, Vmax, is equal to the product of k2, or kcat, and the total concentration of enzyme. The kinetic constant kcat called the turnover number, is the number of substrate molecules converted into product per unit time at a single catalytic site when the enzyme is fully saturated with substrate. Turnover numbers for most enzymes are between 1 and 104 per second. The ratio of kcat/KM provides a measure of enzyme efficiency and specificity.
Allosteric enzymes constitute an important class of enzymes whose catalytic activity can be regulated. These enzymes, which do not conform to Michaelis–
8.5 Enzymes Can Be Inhibited by Specific Molecules
Specific small molecules or ions can inhibit even nonallosteric enzymes. In irreversible inhibition, the inhibitor is covalently linked to the enzyme or bound so tightly that its dissociation from the enzyme is very slow. Covalent inhibitors provide a means of mapping the enzyme’s active site. In contrast, reversible inhibition is characterized by a more rapid and less stable interaction between enzyme and inhibitor. A competitive inhibitor prevents the substrate from binding to the active site. It reduces the reaction velocity by diminishing the proportion of enzyme molecules that are bound to substrate. Competitive inhibition can be overcome by raising the substrate concentration. In uncompetitive inhibition, the inhibitor combines only with the enzyme–
The essence of catalysis is selective stabilization of the transition state. Hence, an enzyme binds the transition state more tightly than it binds the substrate. Transition-
8.6 Enzymes Can Be Studied One Molecule at a Time
Many enzymes are now being studied in singulo, at the level of a single molecule. Such studies are important because they yield information that is difficult to obtain in studies of populations of molecules. Single-
245