Chapter 10
Chapter 10
1. The enzyme catalyzes the first step in the synthesis of pyrimidines. It facilitates the condensation of carbamoyl phosphate and aspartate to form N-carbamoylaspartate and inorganic phosphate.
2. The protonated form of histidine probably stabilizes the negatively charged carbonyl oxygen atom of the scissile bond (bond to be broken) in the transition state. Deprotonation would lead to a loss of activity. Hence, the rate is expected to be half maximal at a pH of about 6.5 (the pK of an unperturbed histidine side chain in a protein) and to decrease as the pH is raised.
3. The inhibition of an allosteric enzyme by the end product of the pathway controlled by the enzyme. It prevents the production of too much end product and the consumption of substrates when product is not required.
4. High concentrations of ATP might signal two overlapping situations. The high levels of ATP might suggest that some nucleotides are available for nucleic acid synthesis, and consequently, UTP and CTP should be synthesized. The high levels of ATP indicate that energy is available for nucleic acid synthesis, and so UTP and CTP should be produced.
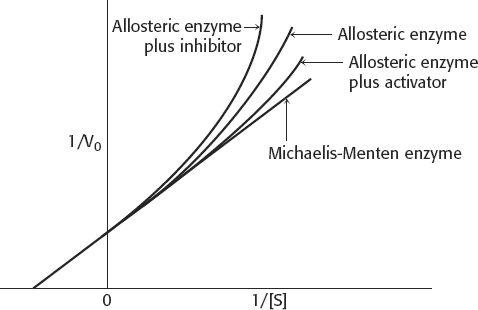
5. All of the enzyme would be in the R form all of the time. There would be no cooperativity. The kinetics would look like that of a Michaelis–
6. The enzyme would show simple Michaelis–
7. CTP is formed by the addition of an amino group to UTP. Evidence indicates the UTP is also capable of inhibiting ATCase in the presence of CTP.
8. Homotropic effectors are the substrates of allosteric enzymes. Heterotropic effectors are the regulators of allosteric enzymes. Homotropic effectors account for the sigmoidal nature of the velocity versus substrate concentration curve, whereas heterotropic effectors alter the KM of the curve. Ultimately, both types of effectors work by altering the T/R ratio.
9. The reconstitution shows that the complex quaternary structure and the resulting catalytic and regulatory properties are ultimately encoded in the primary structure of individual components.
10. If substrates had been used, the enzyme would catalyze the reaction. Intermediates would not accumulate on the enzyme. Consequently, any enzyme that crystallized would have been free of substrates or products.
11. (a) 100. The change in the [R]/[T] ratio on binding one substrate molecule must be the same as the ratio of the substrate affinities of the two forms.
(b) 10. The binding of four substrate molecules changes the [R]/[T] by a factor of 1004 = 108. The ratio in the absence of substrate is 10−7. Hence, the ratio in the fully liganded molecule 108 × 10−7 = 10.
12. The fraction of molecules in the R form is 10−5, 0.004, 0.615, 0.998, and 1 when 0, 1, 2, 3, and 4 ligands, respectively, are bound.
A12
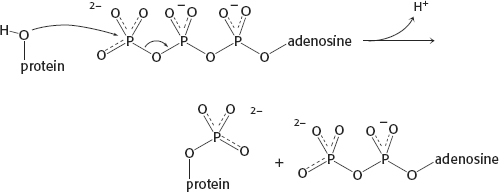
13. The sequential model can account for negative cooperativity, whereas the concerted model cannot.
14.
15. The binding of PALA switches ATCase from the T to the R state because PALA acts as a substrate analog. An enzyme molecule containing bound PALA has fewer free catalytic sites than does an unoccupied enzyme molecule. However, the PALA-
16. The net outcome of the two reactions is the hydrolysis of ATP to ADP and Pi, which has a ΔG of −50 kJ mol−1 (−12 kcal mol−1) under cellular conditions.
17. Isozymes are homologous enzymes that catalyze the same reaction but have different kinetic or regulatory properties.
18. Although the same reaction may be required in a variety of tissues, the biochemical properties of tissues will differ according to their biological function. Isozymes allow the fine-
19. (a) 7; (b) 8; (c) 11; (d) 6; (e) 1; (f ) 12; (g) 3; (h) 4; (i) 5; (j) 2; (k) 10; (l) 9.
20. When phosphorylation takes place at the expense of ATP, sufficient energy is expended to dramatically alter the structure and hence activity of a protein. Moreover, because ATP is the cellular energy currency, protein modification is linked to the energy status of the cell.
21. Covalent modification is reversible, whereas proteolytic cleavage is irreversible.
22. Activation is independent of zymogen concentration because the reaction is intramolecular.
23. Although quite rare, cases of enteropeptidase deficiency have been reported. The affected person has diarrhea and fails to thrive because digestion is inadequate. In particular, protein digestion is impaired.
24. Add blood from the second patient to a sample from the first. If the mixture clots, the second patient has a defect different from that of the first. This type of assay is called a complementation test.
25. Activated factor X remains bound to blood-
26. Antithrombin III is a very slowly hydrolyzed substrate of thrombin. Hence, its interaction with thrombin requires a fully formed active site on the enzyme.
27. Replacing methionine with leucine would be a good choice. Leucine is resistant to oxidation and has nearly the same volume and degree of hydrophobicity as methionine has.
28. Inappropriate clot formation could block arteries in the brain, causing a stroke, or the heart, causing a heart attack.
29. Thrombin catalyzes the hydrolysis of fibrinogen to form active fibrin. But it also has a role in shutting down the cascade by activating protein C, a protease that digests other clotting enzymes Va and VIIIa.
30. Tissue-
31. A mature clot is stabilized by amide linkages between the side chains of lysine and glutamine that are absent in a soft clot. The linkages are formed by transglutaminase.
32. The simple sequential model predicts that the fraction of catalytic chains in the R state, fR, is equal to the fraction containing bound substrate, Y. The concerted model, in contrast, predicts that fR increases more rapidly than Y as the substrate concentration is increased. The change in fR leads to the change in Y on addition of substrate, as predicted by the concerted model.
33. The binding of succinate to the functional catalytic sites of the native c3 moiety changed the visible absorption spectrum of nitrotyrosine residues in the other c3 moiety of the hybrid enzyme. Thus, the binding of substrate analog to the active sites of one trimer altered the structure of the other trimer.
34. According to the concerted model, an allosteric activator shifts the conformational equilibrium of all subunits toward the R state, whereas an allosteric inhibitor shifts it toward the T state. Thus, ATP (an allosteric activator) shifted the equilibrium to the R form, resulting in an absorption change similar to that obtained when substrate is bound. CTP had a different effect. Hence, this allosteric inhibitor shifted the equilibrium to the T form. Thus, the concerted model accounts for the ATP-
35. (a) When crowded, the control group displays gregarious behavior. (b) Inhibition of PKA appears to prevent gregarious behavior, while inhibition of PKG has no effect on the behavior. (c) The effect of PKG inhibition was investigated to establish that the effect seen with PKA inhibition is specific and not just due to the inhibition of any kinase. (d) PKA plays a role in altering behavior of insects. (e) Gregariousness in locusts that are always gregarious is not affected by PKA inhibition, suggesting that PKA may play a role in modifying behavior patterns but not in establishing the patterns.
36. Residues a and d are located in the interior of an α-helical coiled coil, near the axis of the superhelix. Hydrophobic interactions between these side chains contribute to the stability of the coiled coil.
37. In the R state, ATCase expands and becomes less dense. This decrease in density results in a decrease in the sedimentation value.
38. The interaction between trypsin and the inhibitor is so stable that the transition state is rarely formed. Recall that maximal binding energy is released when an enzyme binds to the transition state. If the substrate-
39. Dicoumarol is a competitive inhibitor of γ-glutamyl carboxylase. Consequently, γ-carboxyglutamate, which is required for prothrombin to be converted into thrombin, is not formed. Amino acid composition is determined by hydrolyzing the protein at elevated temperatures in the presence of strong acid. Under these conditions, the carboxyl group of γ-carboxyglutamate is removed from the normal prothrombin, leaving simply glutamate.
A13
40.
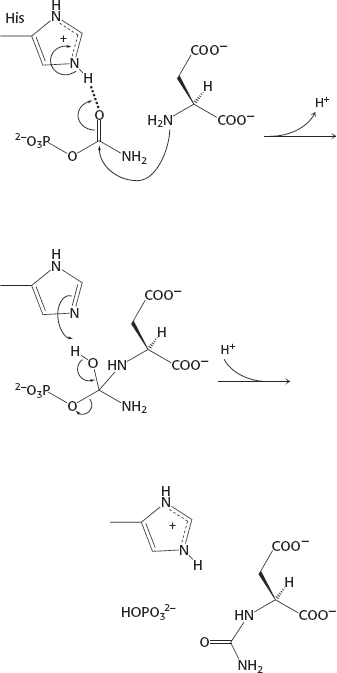
41.