Chapter 15
Chapter 15
1. The highly integrated biochemical reactions that take place inside the cell.
2. Anabolism is the set of biochemical reactions that use energy to build new molecules and ultimately new cells. Catabolism is the set of biochemical reactions that extract energy from fuel sources or break down biomolecules.
3. You reply that vandalism is disrespectful and expensive. Part of your tuition money will now have to pay to remove the vandalism. Plus, the fool should know that Gibbs free energy is at a minimum when a system is in equilibrium.
4. Cellular movements and the performance of mechanical work; active transport; biosynthetic reactions.
5. 1. f; 2. h; 3. i; 4. a; 5. g; 6. b; 7. c; 8. e; 9. j; 10. d.
6. These ions neutralize the charges on the ATP and also facilitate interactions with macromolecules that bind ATP.
7. Charge repulsion, resonance stabilization, increase in entropy, and stabilization by hydration.
8. Trick question. The answer is not known. Adenine appears to form more readily under prebiotic conditions, so ATP may have predominated initially.
9. Having only one nucleotide represent the available energy allows the cell to better monitor its energy status.
10. Increasing the concentration of ATP or decreasing the concentration cellular ADP or Pi (by rapid removal by other reactions, for instance) would make the reaction more exergonic. Likewise, altering the Mg2+ concentration could raise or lower the ΔG of the reaction.
11. The free-
12. Reactions in parts a and c, to the left; reactions in parts b and d, to the right.
13. None whatsoever.
14. (a) ΔG°′ = + 31.4 kJ mol−1 (+7.5 kcal mol−1) and 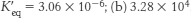 .
.
15. ΔG°′ = +7.1 kJ mol−1 (+1.7 kcal mol−1). The equilibrium ratio is 17.5.
16. (a) Acetate + CoA + H+ goes to acetyl CoA + H2O, ΔG°′ = −31.4 kJ mol−1 (−7.5 kcal mol−1). ATP hydrolysis to AMP and PPi, ΔG°′ = −45.6 kJ mol−1 (−10.9 kcal mol−1). Overall reaction, ΔG°′ = −14.2 kJ mol−1 (−3.4 kcal mol−1).
(b) With pyrophosphate hydrolysis, ΔG°′ = −33.4 kJ mol−1 (−7.98 kcal mol−1). Pyrophosphate hydrolysis makes the overall reaction even more exergonic.
17. (a) For an acid AH,
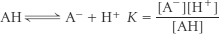
The pK is defined as pK = −log10 K. ΔG°′ is the standard free-
(b) ΔG°′ = +27.32 kJ mol−1 (+6.53 kcal mol−1).
18. Arginine phosphate in invertebrate muscle, like creatine phosphate in vertebrate muscle, serves as a reservoir of high-
19. An ADP unit.
20. (a) The rationale behind creatine supplementation is that it would be converted into creatine phosphate and thus serve as a rapid means of replenishing ATP after muscle contraction. (b) If creatine supplementation is beneficial, it would affect activities that depend on short bursts of activity; any sustained activity would require ATP generation by fuel metabolism, which, as Figure 15.7 shows, requires more time.
21. Under standard conditions, ΔG°′ = − RT ln [products]/[reactants]. Substituting + 23.8 kJ mol−1 (+ 5.7 kcal mol−1) for ΔG°′ and solving for [products]/[reactants] yields 9.9 × 10−5. In other words, the forward reaction does not take place to a significant extent. Under intracellular conditions, ΔG is −1.3 kJ mol−1 (−0.3 kcal mol−1). Using the equation ΔG = ΔG°′ + RT ln [products]/[reactants] and solving for [products]/[reactants] gives a ratio of 5.96 × 10−5. Thus, a reaction that is endergonic under standard conditions can be converted into an exergonic reaction by maintaining the [products]/[reactants] ratio below the equilibrium value. This conversion is usually attained by using the products in another coupled reaction as soon as they are formed.
22. Under standard conditions,
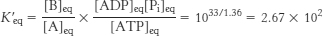
At equilibrium, the ratio of [B] to [A] is given by
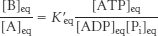
The ATP-
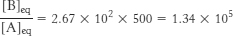
A19
This equilibrium ratio is strikingly different from the value of 1.15 × 10−3 for the reaction A → B in the absence of ATP hydrolysis. In other words, coupling the hydrolysis of ATP with the conversion of A into B has changed the equilibrium ratio of B to A by a factor of about 108.
23. Liver: −45.2 kJ mol−1 (−10.8 kcal mol−1); muscle: −48.1 kJ mol−1 (−11.5 kcal mol−1); brain: −48.5 kJ mol−1 (−11.6 kcal mol−1). The ΔG is most negative in brain cells.
24. (a) Ethanol; (b) lactate; (c) succinate; (d) isocitrate; (e) malate.
25. Recall that ΔG = ΔG°′ + RT ln [products]/[reactants]. Altering the ratio of products to reactants will cause ΔG to vary. In glycolysis, the concentrations of the components of the pathway result in a value of ΔG greater than that of ΔG°′.
26. Higher organisms cannot make vitamins, and thus are dependent on obtaining them from other organisms.
27. Unless the ingested food is converted into molecules capable of being absorbed by the intestine, no energy can ever be extracted by the body.
28. NADH and FADH2 are electron carriers for catabolism; NADPH is the carrier for anabolism.
29. The electrons of the C–
30. Oxidation–
31. Controlling the amount of enzymes; controlling enzyme activity; controlling the availability of substrates.
32. Although the reaction is thermodynamically favorable, the reactants are kinetically stable because of the large activation energy. Enzymes lower the activation energy so that reactions take place on time scales required by the cell.
33. The activated form of sulfate in most organisms is 3′-phosphoadenosine-
34. (a) As the Mg2+ concentration falls, the ΔG of hydrolysis rises. Note that pMg is a logarithmic plot, and so each number on the x-axis represents a 10-
(b) Mg2+ would bind to the phosphates of ATP and help to mitigate charge repulsion. As the [Mg2+] falls, charge stabilization of ATP would be less, leading to greater charge repulsion and an increase in ΔG on hydrolysis.