Chapter 17
Chapter 17
1. The pyruvate dehydrogenase complex catalyzes the following reaction, linking glycolysis and the citric acid cycle:
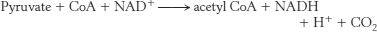
2. Pyruvate dehydrogenase catalyzes the decarboxylation of pyruvate and the formation of acetyllipoamide. Dihydrolipoyl transacetylase catalyzes the formation of acetyl CoA. Dihydrolipoyl dehydrogenase catalyzes the reduction of the oxidized lipoic acid. The kinase associated with the complex phosphorylates and inactivates the complex, whereas the phosphatase dephosphorylates and activates the complex.
3. Thiamine pyrophosphate plays a role in the decarboxylation of pyruvate. Lipoic acid (as lipoamide) transfers the acetyl group. Coenzyme A accepts the acetyl group from lipoic acid to form acetyl CoA. FAD accepts the electrons and hydrogen ions when reduced lipoic acid is oxidized. NAD+ accepts electrons from FADH2.
4. Catalytic coenzymes (TPP, lipoic acid, and FAD) are modified but regenerated in each reaction cycle. Thus, they can play a role in the processing of many molecules of pyruvate. Stoichiometric coenzymes (coenzyme A and NAD+) are used in only one reaction because they are the components of products of the reaction.
5. The remaining steps regenerate oxidized lipoamide, which is required to begin the next reaction cycle. Moreover, this regeneration results in the production of high-
6. The advantages are as follows:
The reaction is facilitated by having the active sites in proximity.
The reactants do not leave the enzyme until the final product is formed.
Constraining the reactants minimizes loss due to diffusion and minimizes side reactions.
All of the enzymes are present in the correct amounts.
Regulation is more efficient because the regulatory enzymes—
7. (a) After one round of the citric acid cycle, the label emerges in C-
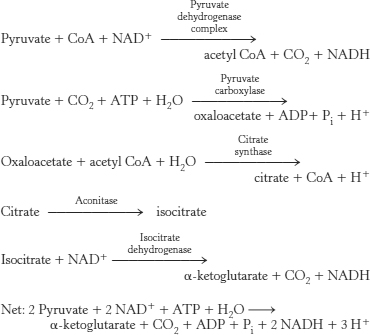
8. (a) Isocitrate lyase and malate synthase are required in addition to the enzymes of the citric acid cycle.
(b) 2 Acetyl CoA + 2 NAD+ + FAD + 3 H2O → oxaloacetate + 2 CoA + 2 NADH + FADH2 + 3 H+.
(c) No. Hence, mammals cannot carry out the net synthesis of oxaloacetate from acetyl CoA.
9. −41.0 kJ mol−1 (−9.8 kcal mol−1)
10. Enzymes or enzyme complexes are biological catalysts. Recall that a catalyst facilitates a chemical reaction without the catalyst itself being permanently altered. Oxaloacetate can be thought of as a catalyst because it binds to an acetyl group, leads to the oxidative decarboxylation of the two carbon atoms, and is regenerated at the completion of a cycle. In essence, oxaloacetate (and any cycle intermediate) acts as a catalyst.
11. Thiamine thiazolone pyrophosphate is a transition-
12. (a) 6; (b) 10; (c) 1; (d) 7; (e) 2; (f) 8; (g) 3; (h) 4; (i) 5; (j) 9.
13. A decrease in the amount of O2 will necessitate an increase in anaerobic glycolysis for energy production, leading to the generation of a large amount of lactic acid. Under conditions of shock, the kinase inhibitor is administered to ensure that pyruvate dehydrogenase is operating maximally.
14. (a) As is stated in the previous problem, DCA inhibits pyruvate dehydrogenase kinase.
(b) The fact that inhibiting the kinase results in more dehydrogenase activity suggests that there must be some residual activity that is being inhibited by the kinase.
15. Acetyllipoamide and acetyl CoA.
16. In muscle, the acetyl CoA generated by the complex is used for energy generation. Consequently, signals that indicate an energy-
17. (a) Enhanced kinase activity will result in a decrease in the activity of the PDH complex because phosphorylation by the kinase inhibits the complex.
(b) Phosphatase activates the complex by removing a phosphate. If the phosphatase activity is diminished, the activity of the PDH complex also will decrease.
18. She might have been ingesting, in some fashion, the arsenite from the peeling paint or the wallpaper. Also, she might have been breathing arsine gas from the wallpaper, which would be oxidized to arsenite in her body. In any of these circumstances, the arsenite inhibited enzymes that require lipoic acid—
19. (a) 5; (b) 7; (c) 1; (d) 10; (e) 2; (f) 4; (g) 9; (h) 3; (i) 8; (j) 6.
20. The TCA cycle depends on a steady supply of NAD+ as an oxidant, generating NADH. O2 is never directly utilized in the cycle. However, NAD+ is regenerated via donation of electrons to O2 by way of the electron transport chain, so eventually a lack of O2 will cause the cycle to cease due to a lack of NAD+.
A22
21. Succinate dehydrogenase is the only enzyme in the citric acid cycle that is embedded in the mitochondrial membrane, which makes it associated with the electron-
22. (a) The steady-
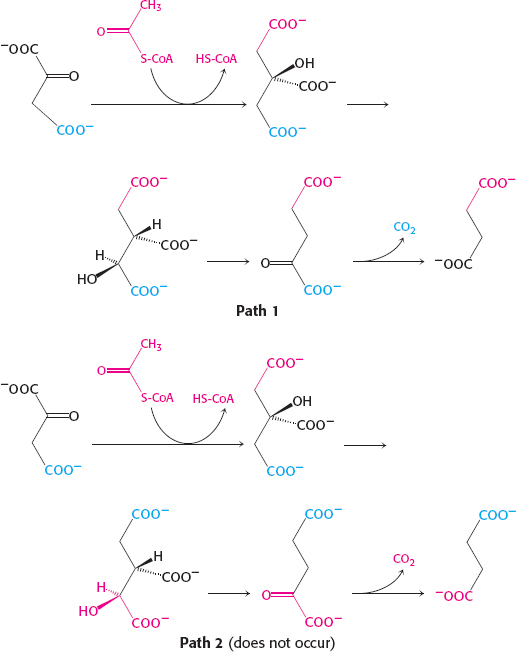
23.
24. Succinate will increase in concentration, followed by α-ketoglutarate and the other intermediates “upstream” of the site of inhibition. Succinate has two methylene groups that are required for the dehydrogenation, whereas malonate has but one.
25. Pyruvate carboxylase should be active only when the acetyl CoA concentration is high. Acetyl CoA might accumulate if the energy needs of the cell are not being met, because of a deficiency of oxaloacetate. Under these conditions the pyruvate carboxylase catalyzes an anaplerotic reaction. Alternatively, acetyl CoA might accumulate because the energy needs of the cell have been met. In this circumstance, pyruvate will be converted back into glucose, and the first step in this conversion is the formation of oxaloacetate.
26. The energy released when succinate is oxidized to fumarate is not sufficient to power the synthesis of NADH but is sufficient to reduce FAD.
27. Citrate is a tertiary alcohol that cannot be oxidized, because oxidation requires a hydrogen atom to be removed from the alcohol and a hydrogen atom to be removed from the carbon atom bonded to the alcohol. No such hydrogen exists in citrate. The isomerization converts the tertiary alcohol into isocitrate, which is a secondary alcohol that can be oxidized.
28. The enzyme nucleoside diphosphokinase transfers a phosphoryl group from GTP (or any nucleoside triphosphate) to ADP according to the reversible reaction:

29. The reaction is powered by the hydrolysis of a thioester. Acetyl CoA provides the thioester that is converted into citryl CoA. When this thioester is hydrolyzed, citrate is formed in an irreversible reaction.
30. It enables organisms such as plants and bacteria to convert fats, through acetyl CoA, into glucose.
31. We cannot get the net conversion of fats into glucose, because the only means to get the carbon atoms from fats into oxaloacetate, the precursor of glucose, is through the citric acid cycle. However, although two carbon atoms enter the cycle as acetyl CoA, two carbon atoms are lost as CO2 before oxaloacetate is formed. Thus, although some carbon atoms from fats may end up as carbon atoms in glucose, we cannot obtain a net synthesis of glucose from fats.
32. Acetyl CoA will inhibit the complex. Glucose metabolism to pyruvate will be slowed because acetyl CoA is being derived from an alternative source.
33. The enol intermediate of acetyl CoA attacks the carbonyl carbon atom of glyoxylate to form a C–
34. Citrate is a symmetric molecule. Consequently, the investigators assumed that the two –CH2COO− groups in it would react identically. Thus, for every citrate molecule undergoing the reactions shown in path 1, they thought that another citrate molecule would react as shown in path 2. If so, then only half the label should have emerged in the CO2.
35. Call one hydrogen atom A and the other B. Now suppose that an enzyme binds three groups of this substrate—
A23
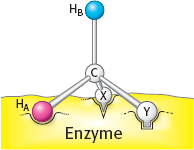
Sterically nonequivalent groups such as HA and HB will almost always be distinguished in enzymatic reactions. The essence of the differentiation of these groups is that the enzyme holds the substrate in a specific orientation. Attachment at three points, as depicted in the diagram, is a readily visualized way of achieving a particular orientation of the substrate, but it is not the only means of doing so.
36. (a) The complete oxidation of citrate requires 4.5 μmol of O2 for every micromole of citrate.
C6H8O7 + 4.5 O2 → 6 CO2 + 4 H2O
Thus, 13.5 μmol of O2 would be consumed by 3 μmol of citrate.
(b) Citrate led to the consumption of far more O2 than can be accounted for simply by the oxidation of citrate itself. Citrate thus facilitated O2 consumption.
37. (a) In the absence of arsenite, the amount of citrate remained constant. In its presence, the concentration of citrate fell, suggesting that it was being metabolized.
(b) The action of arsenite is not altered. Citrate still disappears.
(c) Arsenite is preventing the regeneration of citrate. Recall that arsenite inhibits the pyruvate dehydrogenase complex.
38. (a) The initial infection is unaffected by the absence of isocitrate lyase, but the absence of this enzyme inhibits the latent phase of the infection.
(b) Yes.
(c) A critic could say that, in the process of deleting the isocitrate lyase gene, some other gene was damaged, and it is the absence of this other gene that prevents latent infection. Reinserting the isocitrate lyase gene into the bacteria from which it had been removed renders the criticism less valid.
(d) Isocitrate lyase enables the bacteria to synthesize carbohydrates that are necessary for survival, including carbohydrate components of the cell membrane.