Chapter 2
Chapter 2
1. (A) Proline, Pro, P; (B) tyrosine, Tyr, Y; (C) leucine, Leu, L; (D) lysine, Lys, K.
2. (a) C, B, A; (b) D; (c) D, B; (d) B, D; (e) B.
3. (a) 6; (b) 2; (c) 3; (d) 1; (e) 4; (f ) 5.
4. (a) Ala; (b) Tyr; (c) Ser; (d) His.
5. Ser, Glu, Tyr, Thr
6. (a) Alanine-
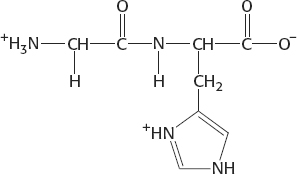
7. At pH 5.5, the net charge is +1:
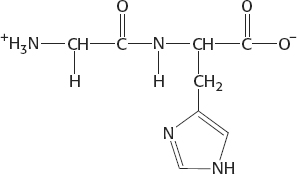
At pH 7.5, the net charge is 0:
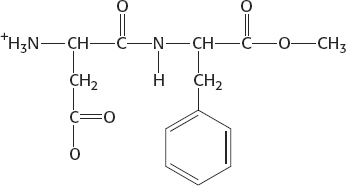
8. There are 20 choices for each of the 50 amino acids: 2050, or 1 × 1065.
9.
10. The (nitrogen–
11. Side chain is the functional group attached to the α-carbon atom of an amino acid.
12. Amino acid composition refers simply to the amino acids that make up the protein. The order is not specified. Amino acid sequence is the same as the primary structure—
13. (a) Each strand is 35 kDa and hence has about 318 residues (the mean residue mass is 110 daltons). Because the rise per residue in an α helix is 1.5 Å, the length is 477 Å. More precisely, for an α-helical coiled coil, the rise per residue is 1.46 Å; so the length is 464 Å. (b) Eighteen residues in each strand (40 minus 4 divided by 2) are in a β-sheet conformation. Because the rise per residue is 3.5 Å, the length is 63 Å.
14. The methyl group attached to the β-carbon atom of isoleucine sterically interferes with α-helix formation. In leucine, this methyl group is attached to the γ-carbon atom, which is farther from the main chain and hence does not interfere.
A2
15. Proline and glycine. The cyclic side chain of proline linking the nitrogen and α-carbon atoms limits ϕ to a very narrow range (around −60 degrees). The lack of steric hindrance exhibited by the side chain hydrogen atom of glycine enables this amino acid to access a much greater area of the Ramachandran plot.
16. The first mutation destroys activity because valine occupies more space than alanine does, and so the protein must take a different shape, assuming that this residue lies in the closely packed interior. The second mutation restores activity because of a compensatory reduction of volume; glycine is smaller than isoleucine.
17. Loops invariably are on the surface of proteins, exposed to the environment. Because many proteins exist in aqueous environments, the exposed loops will be hydrophilic to interact with water.
18. The native conformation of insulin is not the thermodynamically most stable form, because it contains two separate chains linked by disulfide bonds. Insulin is formed from proinsulin, a single-
19. A segment of the main chain of the protease could hydrogen bond to the main chain of the substrate to form an extended parallel or antiparallel pair of β strands.
20. Glycine has the smallest side chain of any amino acid. Its size is often critical in allowing polypeptide chains to make tight turns or to approach one another closely.
21. Glutamate, aspartate, and the terminal carboxylate can form salt bridges with the guanidinium group of arginine. In addition, this group can be a hydrogen-
22. Disulfide bonds in hair are broken by adding a thiol-
23. Some proteins that span biological membranes are “the exceptions that prove the rule” because they have the reverse distribution of hydrophobic and hydrophilic amino acids. For example, consider porins, proteins found in the outer membranes of many bacteria. Membranes are built largely of hydrophobic chains. Thus, porins are covered on the outside largely with hydrophobic residues that interact with the neighboring hydrophobic chains. In contrast, the center of the protein contains many charged and polar amino acids that surround a water-
24. The amino acids would be hydrophobic in nature. An α helix is especially suited to crossing a membrane because all of the amide hydrogen atoms and carbonyl oxygen atoms of the peptide backbone take part in intrachain hydrogen bonds, thus stabilizing these polar atoms in a hydrophobic environment.
25. This example demonstrates that the pKa values are affected by the environment. A given amino acid can have a variety of pKa values, depending on the chemical environment inside the protein.
26. Recall that hemoglobin exists as a tetramer while myoglobin is a monomer. Consequently, the hydrophobic residues on the surface of hemoglobin subunits are probably involved in van der Waals interactions with similar regions on the other subunits, and will be shielded from the aqueous environment by this interaction.
27. A possible explanation is that the severity of the symptoms corresponds to the degree of structural disruption. Hence, substitution of alanine for glycine might result in mild symptoms, but substitution of the much larger tryptophan may result in little or no collagen triple-
28. The energy barrier that must be crossed to go from the polymerized state to the hydrolyzed state is large even though the reaction is thermodynamically favorable.
29. Using the Henderson–
30. The assignment of absolute configuration requires the assignment of priorities to the four groups connected to a tetrahedral carbon atom. For all amino acids except cysteine, the priorities are: (1) amino group; (2) carbonyl group; (3) side chain; (4) hydrogen. For cysteine, because of the sulfur atom in its side chain, the side chain has a greater priority than does the carbonyl group, leading to the assignment of an R rather than S configuration.
31. ELVISISLIVINGINLASVEGAS
32. No, Pro–
33. A, c; B, e; C, d; D, a; E, b.
34. The reason is that the wrong disulfides formed pairs in urea. There are 105 different ways of pairing eight cysteine molecules to form four disulfides; only one of these combinations is enzymatically active. The 104 wrong pairings have been picturesquely termed “scrambled” ribonuclease.