Chapter 21
Chapter 21
1. Step 1 is the release of glucose 1-
2. (a) 8; (b) 3; (c) 6; (d) 5; (e) 9; (f) 2; (g) 10; (h) 1; (i) 4; (j) 7.
3. Glycogen is an important fuel reserve for several reasons. The controlled breakdown of glycogen and release of glucose increase the amount of glucose that is available between meals. Hence, glycogen serves as a buffer to maintain blood-
4. As an unbranched polymer, α-amylose has only one nonreducing end. Therefore, only one glycogen phosphorylase molecule could degrade each α-amylose molecule. Because glycogen is highly branched, there are many nonreducing ends per molecule. Consequently, many phosphorylase molecules can release many glucose molecules per glycogen molecule.
A28
5. The patient has a deficiency of the branching enzyme.
6. In muscle, the b form of phosphorylase is activated by AMP. In the liver, the a form is inhibited by glucose. The difference corresponds to the difference in the metabolic role of glycogen in each tissue. Muscle uses glycogen as a fuel for contraction, whereas the liver uses glycogen to maintain blood-
7. Cells maintain the [Pi]/[glucose 1-
8. The high level of glucose 6-
9. The phosphoryl donor is glucose 1,6-
10. The different manifestations correspond to the different roles of the liver and muscle. Liver glycogen phosphorylase plays a crucial role in the maintenance of blood-
11. Water is excluded from the active site to prevent hydrolysis. The entry of water could lead to the formation of glucose rather than glucose 1-
12. The amylase activity was necessary to remove all of the glycogen from the glycogenin. Recall that glycogenin synthesizes oligosaccharides of about eight glucose units, and then activity stops. Consequently, if the glucose residues are not removed by extensive amylase treatment, glycogenin will not function.
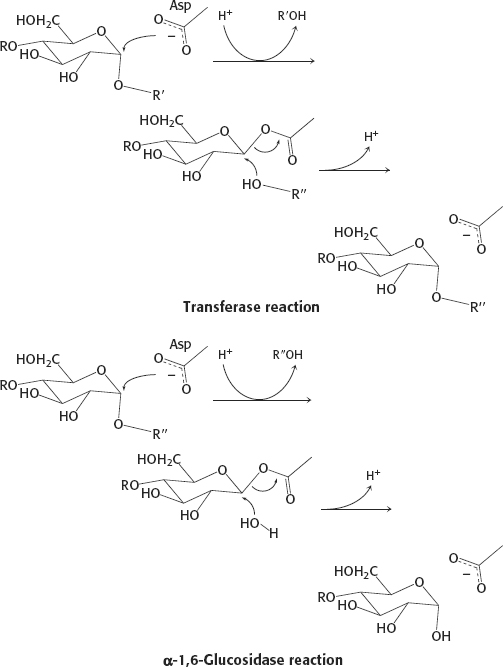
13. The substrate can be handed directly from the transferase site to the debranching site.
14. During exercise, [ATP] falls and [AMP] rises. Recall that AMP is an allosteric activator of glycogen phosphorylase b. Thus, even in the absence of covalent modification by phosphorylase kinase, glycogen is degraded.
15. Although glucose 1-
16. Epinephrine binds to its G-
17. First, the signal-
18. It prevents both from operating simultaneously, which would lead to a useless expenditure of energy.
19. All these symptoms suggest central nervous system problems. If exercise is exhaustive enough or the athlete has not prepared well enough or both, liver glycogen also can be depleted. The brain depends on glucose derived from liver glycogen. The symptoms suggest that the brain is not getting enough fuel (hypoglycemia).
20. Liver phosphorylase a is inhibited by glucose, which facilitates the R → T transition. This transition releases PP1, which inactivates glycogen breakdown and stimulates glycogen synthesis. Muscle phosphorylase is insensitive to glucose.
21. (a) 4; (b) 1; (c) 5; (d) 10; (e) 7; (f) 2; (g) 8; (h) 9; (i) 6; (j) 3.
22. Phosphoglucomutase, UDP-
23. The enzyme pyrophosphatase converts the pyrophosphate into two molecules of inorganic phosphate. This conversion renders the overall reaction irreversible.
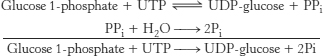
24. The presence of high concentrations of glucose 6-
25. Free glucose must be phosphorylated at the expense of a molecule of ATP. Glucose 6-
26. Breakdown: Phosphoglucomutase converts glucose 1-
27.
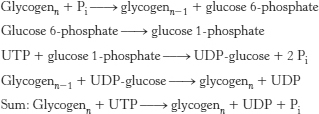
28. In principle, having glycogen be the only primer for the further synthesis of glycogen should be a successful strategy. However, if the glycogen granules were not evenly divided between daughter cells, glycogen stores for future generations of cells might be compromised. Glycogenin synthesizes the primer for glycogen synthase.
29. Insulin binds to its receptor and activates the tyrosine kinase activity of the receptor, which in turn triggers a pathway that activates protein kinases. The kinases phosphorylate and inactivate glycogen synthase kinase. Protein phosphatase 1 then removes the phosphate from glycogen synthase and thereby activates the synthase.
A29
30.
31. In the liver, glucagon stimulates the cAMP-
32.
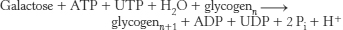 .
.
33. Phosphorylase, transferase, glucosidase, phosphoglucomutase, and glucose 6-
34. Glucose is an allosteric inhibitor of phosphorylase a. Hence, crystals grown in its presence are in the T state. The addition of glucose 1-
35. Galactose is converted into UDP-
36. This disease can also be produced by a mutation in the gene that encodes the glucose 6-
37. (a) Apparently, the glutamate, with its negatively charged R group, can mimic to some extent the presence of a phosphoryl group on serine. That the stimulation is not as great is not surprising in that the carboxyl group is smaller and not as charged as the phosphate. (b) Substitution of aspartate would give some stimulation, but because it is smaller than the glutamate, the stimulation would be smaller.
38. (a) Glycogen was too large to enter the gel and, because analysis was by western blot with the use of an antibody specific to glycogenin, we would not expect to see background proteins.
(b) α-Amylase degrades glycogen, releasing the protein glycogenin, which can be visualized by a western blot.
(c) Glycogen phosphorylase, glycogen synthase, and protein phosphatase 1. These proteins might be visible if the gel were stained for protein, but a western analysis reveals the presence of glycogenin only.
39. (a) The smear was due to molecules of glycogenin with increasingly large amounts of glycogen attached to them.
(b) In the absence of glucose in the medium, glycogen is metabolized, resulting in a loss of the high-
(c) Glycogen could have been resynthesized and added to the glycogenin when the cells were fed glucose again.
(d) No difference between lanes 3 and 4 suggests that, by 1 hour, the glycogen molecules had attained maximum size in this cell line. Prolonged incubation does not apparently increase the amount of glycogen.
(e) α-Amylase removes essentially all of the glycogen, and so only the glycogenin remains.