Chapter 24
Chapter 24
1. Nitrogen fixation is the conversion of atmospheric N2 into NH4+. Diazotrophic (nitrogen-
2. (a) 4; (b) 8; (c) 10; (d) 6; (e) 7; (f) 9; (g) 3; (h) 5; (i) 2; (j) 1.
3. The reductase provides electrons with high reducing power, whereas the nitrogenase, which requires ATP hydrolysis, uses the electrons to reduce N2 to NH3.
4. False. Nitrogen is thermodynamically favored. ATP expenditure by the nitrogenase is required to make the reaction kinetically possible.
5. The bacteria provide the plant with ammonia by reducing atmospheric nitrogen. This reduction is energetically expensive, and the bacteria use ATP from the plant.
6. Oxaloacetate, pyruvate, ribose-
7. Human beings do not have the biochemical pathways to synthesize certain amino acids from simpler precursors. Consequently, these amino acids are “essential” and must be obtained from the diet.
A35
8. Glucose + 2 ADP + 2 Pi + 2 NAD+ + 2 glutamate → 2 alanine + 2 α-ketoglutrate + 2 ATP + 2 NADH + 2 H2O + 2 H+.
9. N2 → NH4+ → glutamate → serine → glycine → δ-aminolevulinate → porphobilinogen → heme.
10. Pyridoxal phosphate (PLP).
11. S-Adenosylmethionine, tetrahydrofolate, and methylcobalamin.
12. (a) N5,N10-Methylenetetrahydrofolate; (b) N5-methyltetrahydrofolate.
13. γ-Glutamyl phosphate is a likely reaction intermediate.
14. The synthesis of asparagine from aspartate passes through an acyl-
15. The administration of glycine leads to the formation of isovalerylglycine. This water-
16. The nitrogen atom shaded red is derived from glutamine. The carbon atom shaded blue is derived from serine.
17. They carry out nitrogen fixation. The absence of photosystem II provides an environment in which O2 is not produced. Recall that the nitrogenase is very rapidly inactivated by O2.
18. The cytoplasm is a reducing environment, whereas the extracellular milieu is an oxidizing environment.
19. (a) None; (b) d-glutamate and oxaloacetate.
20. Succinyl CoA is formed in the mitochondrial matrix as part of the citric acid cycle.
21. Alanine from pyruvate; aspartate from oxaloacetate; glutamate from α-ketoglutarate.
22. Lysine cyclodeaminase converts l-lysine into the six-
23. Y could inhibit the C → D step, Z could inhibit the C → F step, and C could inhibit A → B. This scheme is an example of sequential feedback inhibition. Alternatively, Y could inhibit the C → D step, Z could inhibit the C → F step, and the A → B step would be inhibited only in the presence of both Y and Z. This scheme is called concerted feedback inhibition.
24. The rate of the A → B step in the presence of high levels of Y and Z would be 24 s−1 (0.6 × 0.4 × 100 s−1).
25. Lysine 258 is absolutely essential for the activity of aspartate aminotransferase, as it is responsible both for the formation of the internal aldimine with the pyridoxal phosphate cofactor and for transferring the proton between the ketimine and quinonoid intermediates. Mutation of this residue to cysteine would be expected to dramatically impair catalysis, as cysteine cannot occupy the same space as lysine and also exhibits differing pKa properties. Upon treatment with 2-
26. An external aldimine forms with SAM, which is deprotonated to form the quinonoid intermediate. The deprotonated carbon atom attacks the carbon atom adjacent to the sulfur atom to form the cyclopropane ring and release methylthioadenosine, the other product.
27. An external aldimine forms with l-serine, which is deprotonated to form the quinonoid intermediate. This intermediate is reprotonated on its opposite face to form an aldimine with d-serine. This compound is cleaved to release d-serine. The equilibrium constant for a racemization reaction is 1 because the reactant and product are exact mirror images of each other.
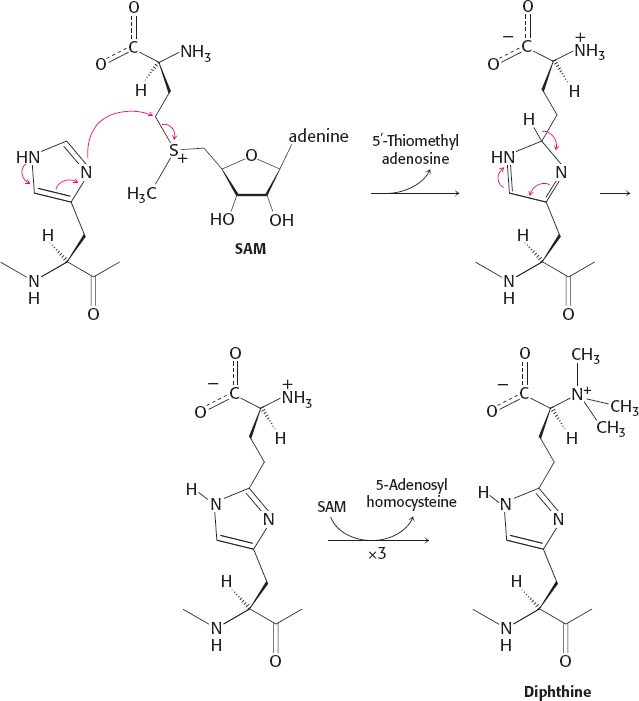
28. (a) In the first step, histidine attacks the methylene group from the methionine subgroup of SAM (rather than the usual methyl substituent), resulting in the transfer of an aminocarboxypropyl group. Three subsequent conventional SAM-
(b) In this chapter, we have observed two examples of an ATP-
29. Biotin.
30. Synthesis from oxaloacetate and α-ketoglutarate would deplete the citric acid cycle, which would decrease ATP production. Anapleurotic reactions would be required to replenish the citric acid cycle.
31. SAM is the donor for DNA methylation reactions that protect a host from digestion by its own restriction enzymes. A lack of SAM would render the bacterial DNA susceptible to digestion by the cell’s own restriction enzymes.
A36
32. Acetate → acetyl-
33. The value of KM of glutamate dehydrogenase for NH4+ is high (>> 1 mM), and so this enzyme is not saturated when NH4+ is limiting. In contrast, glutamine synthetase has very high affinity for NH4+. Thus, ATP hydrolysis is required to capture ammonia when it is scarce.
34. (a) Asparagine is much more abundant in the dark. More glutamine is present in the light. These amino acids show the most dramatic effects. Glycine also is more abundant in the light.
(b) Glutamine is a more metabolically reactive amino acid, used in the synthesis of many other compounds. Consequently, when energy is available as light, glutamine will be preferentially synthesized. Asparagine, which carries more nitrogen per carbon atom and is thus a more-
(c) White asparagus has an especially high concentration of asparagine, which accounts for its intense taste. All asparagus has a large amount of asparagine. In fact, as suggested by its name, asparagine was first isolated from asparagus.