Chapter 27
Chapter 27
1. Over the 40 years under consideration, our test subject will have consumed
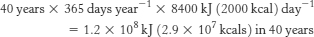
Thus, over the 40-
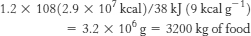
which is equivalent to more than 6 tons of food!
2. 55 pounds = 25 kg = 25,000 g = total weight gain
40 years × 365 days year−1 = 14,600 days
25,000 g/14,600 days = 1.7 g day−1
which is equivalent to an extra pat of butter per day. Her BMI is 26.5, and she would be considered overweight but not obese.
3. Adipose tissue is now known to be an active endocrine organ, secreting signal molecules called adipokines.
4. Caloric homeostasis is the condition in which the energy expenditure of an organism is equal to the energy intake.
5. Leptin and insulin.
6. CCK produces a feeling of satiety and stimulates the secretion of digestive enzymes by the pancreas and the secretion of bile salts by the gall bladder. GLP-
A40
7. Obviously, something is amiss. Although the answer is not known, the leptin-
8. 1: a, b; 2: f; 3: c, d, f; 4: c, d; 5: c; 6: f; 7: e; 8: e; 9: e.
9. Phosphorylation of dietary glucose after it enters the liver; gluconeogenesis; glycogen breakdown.
10. Type 1 diabetes is due to autoimmune destruction of the insulin-
11. Leptin stimulates processes impaired in diabetes. For instance, leptin stimulates fatty acid oxidation, inhibits triacylglycerol synthesis, and increases the sensitivity of muscle and the liver to insulin.
12. (a) A watt is equal to 1 joule (J) per second (0.239 calorie per second). Hence, 70 W is equivalent to 0.07 kJ s−1 (0.017 kcal s−1).
(b) A watt is a current of 1 ampere (A) across a potential of 1 volt (V). For simplicity, let us assume that all the electron flow is from NADH to O2 (a potential drop of 1.14 V). Hence, the current is 61.4 A, which corresponds to 3.86 × 1020 electrons per second (1 A = 1 coulomb s−1 = 6.28 = 1018 charge s−1).
(c) About 2.5 molecules of ATP are formed per molecule of NADH oxidized (two electrons). Hence, 1 molecule of ATP is formed per 0.8 electron transferred. A flow of 3.86 × 1020 electrons per second therefore leads to the generation of 4.83 × 1020 molecules of ATP per second, or 0.80 mmol s−1.
(d) The molecular weight of ATP is 507. The total body content of ATP of 50 g is equal to 0.099 mol. Hence, ATP turns over about once in 125 seconds when the body is at rest.
13. The stoichiometry of the complete oxidation of glucose is C6H12O6 + 6 O2 → 6CO2 + 6 H2O
and that of tripalmitoylglycerol is
C51H98O2 + 72.5 O2 → 51 CO2 + 49 H2O
Hence, the RQ values are 1.0 and 0.703, respectively.
An RQ value reveals the relative use of carbohydrates and fats as fuels. The RQ of a marathon runner typically decreases from 0.97 to 0.77 in the course of a race. The lowering of the RQ indicates the shift in fuel from carbohydrates to fat.
14. One gram of glucose (molecular weight 180.2) is equal to 5.55 mmol, and one gram of tripalmitoylglycerol (molecular weight 807.3) is equal to 1.24 mmol. The reaction stoichiometries (Problem 13) indicate that 6 mol of H2O is produced per mole of glucose oxidized, and 49 mol of H2O is produced per mole of tripalmitoylglycerol oxidized. Hence, the H2O yields per gram of fuel are 33.3 mmol (0.6 g) for glucose and 60.8 mmol (1.09 g) for tripalmitoylglycerol. Thus, complete oxidation of this fat gives 1.82 times as much water as does glucose. Another advantage of triacylglycerols is that they can be stored in essentially anhydrous form, whereas glucose is stored as glycogen, a highly hydrated polymer. A hump consisting mainly of glycogen would be an intolerable burden—
15. The starved–
16. Ethanol is oxidized to yield acetaldehyde by alcohol dehydrogenase, which is subsequently oxidized to acetate and acetaldehyde. Ethanol is also metabolized to acetaldehyde by the P450 enzymes, with the subsequent depletion of NADPH.
17. First, fatty liver develops owing to the increased amounts of NADH that inhibit fatty acid oxidation and stimulate fatty acid synthesis. Second, alcoholic hepatitis begins owing to oxidative damage and damage due to excess acetaldehyde that results in cell death. Finally, fibrous tissues form, creating scars that impair blood flow and biochemical function. Ammonia cannot be converted into urea, and its toxicity leads to coma and death.
18. A typical macadamia nut has a mass of about 2 g. Because it consists mainly of fats (~37 kJ g−1, ~9 kcal g−1), a nut has a value of about 75 kJ (18 kcal). The ingestion of 10 nuts results in an intake of about 753 kJ (180 kcal). As stated in the answer to Problem 12, a power consumption of 1 W corresponds to 1 J s−1 (0.239 cal s−1), and so 400-
19. A high blood-
20. A lack of adipose tissue leads to an accumulation of fats in the muscle, with the generation of insulin resistance. The experiment shows that adipokines secreted by the adipose tissue, here leptin, facilitate in some fashion the action of insulin in muscle.
21. Such a mutation would increase the phosphorylation of the insulin receptor and IRS in muscle and would improve insulin sensitivity. Indeed, PTP1B is an attractive therapeutic target for type 2 diabetes.
22. Lipid mobilization can be so rapid that it exceeds the ability of the liver to oxidize the lipids or convert them into ketone bodies. The excess is reesterified and released into the blood as VLDLs.
23. A role of the liver is to provide glucose for other tissues. In the liver, glycolysis is used not for energy production but for biosynthetic purposes. Consequently, in the presence of glucagon, liver glycolysis stops so that the glucose can be released into the blood.
24. The urea cycle and gluconeogenesis.
25. (a) Insulin inhibits lipid utilization.
(b) Insulin stimulates protein synthesis, but there are no amino acids in the children’s diet. Moreover, insulin inhibits protein breakdown. Consequently, muscle proteins cannot be broken down and used for the synthesis of essential proteins.
(c) Because proteins cannot be synthesized, blood osmolarity is too low. Consequently, fluid leaves the blood. An especially important protein for maintaining blood osmolarity is albumin.
26. During strenuous exercise, muscle converts glucose into pyruvate through glycolysis. Some of the pyruvate is processed by cellular respiration. However, some of it is converted into lactate and released into the blood. The liver takes up the lactate and converts it into glucose through gluconeogenesis. Muscle may process the carbon skeletons of branched-
27. This conversion allows muscle to function anaerobically. NAD+ is regenerated when pyruvate is reduced to lactate, and so energy can continue to be extracted from glucose during strenuous exercise. The liver converts the lactate into glucose.
A41
28. Fatty acids and glucose, respectively.
29. This practice is called carbo-
30. The oxygen consumption at the end of exercise is used to replenish ATP and creatine phosphate and to oxidize any lactate produced.
31. Oxygen is used in oxidative phosphorylation to resynthesize ATP and creatine phosphate. The liver converts lactate released by the muscle into glucose. Blood must be circulated to return the body temperature to normal, and so the heart cannot return to its resting rate immediately. Hemoglobin must be reoxygenated to replace the oxygen used in exercise. The muscles that power breathing must continue working at the same time as the exercised muscles are returning to resting states. In essence, all the biochemical systems activated in intense exercise need increased oxygen to return to the resting state.
32. Ethanol may replace water that is hydrogen bonded to proteins and membrane surfaces. This alteration of the hydration state of the protein would alter its conformation and hence function. Ethanol may also alter phospholipid packing in membranes. The two effects suggest that integral membrane proteins would be most sensitive to ethanol, as indeed seems to be the case.
33. Cells from the type I fiber would be rich in mitochondria, whereas those of the type II fiber would have few mitochondria.
34. (a) The ATP expended during this race amounts to about 8380 kg, or 18,400 pounds. (b) The cyclist would need about $1,260,000,000 to complete the race.
35. Exercise greatly enhances the ATP needs of muscle cells. To more efficiently meet these needs, more mitochondria are synthesized.
36. AMPK activity increases as ATP is used for muscle contraction. AMPK inactivates acetyl CoA carboxylase. Recall that malonyl CoA, the product of acetyl CoA carboxylase, inhibits transport of fatty acids into the mitochondria. The decrease in muscle malonyl CoA allows fatty acid oxidation in muscle.
37. After the first several days of starvation, most tissues are using fatty acids. Ketone bodies provide much of the brain’s energy, decreasing glucose requirement. The low insulin/high glucagon ration stimulates lipolysis and gluconeogenesis. However, gluconeogenesis will not occur because the increase in fatty acid oxidation increases acetyl CoA and NADH. The high concentration of NADH inhibits the TCA cycle as well as gluconeogenesis. The acetyl CoA forms ketone bodies.
38. (a) The increase in all of the ratios is due to the NADH glut caused by the metabolism of alcohol.
(b) The increased amounts of lactate and d-3-
(c) Drinking on an empty stomach suggests that glycogen stores are low. Because of the NADH glut, gluconeogenesis cannot occur. Consequently, hypoglycemia results. A well-
39. If glucose is always provided, even in small amounts, the brain will use glucose as a fuel rather than adapting to ketone body use. During the fast, muscle protein will be broken down to meet the brains glucose needs. This protein degradation will lead to organ failure sooner than if the brain had adapted to ketone body utilization.
40. The inability of muscle mitochondria to process all of the fatty acids produced by overnutrition leads to excessive levels of diacylglycerol and ceramide in the muscle cytoplasm. These second-
41. Both are due to a lack of thiamine (vitamin B1). Thiamine, which is sometimes called aneurin, is required most notably for the proper functioning of pyruvate dehydrogenase.
42. (a) Red blood cells always produce lactate, and fast-
(b) At that point, the athlete is beginning to move into anaerobic exercise, in which most energy is produced by anaerobic glycolysis.
(c) The lactate threshold is essentially the point at which the athlete switches from aerobic exercise, which can be done for extended periods, to anaerobic exercise, essentially sprinting, which can be done for only short periods. The idea is to race at the extreme of his or her aerobic capacity until the finish line is in sight and then to switch to anaerobic.
(d) Training increases the amount of blood vessels and the number of muscle mitochondria. Together, they increase the ability to process glucose aerobically. Consequently, a greater effort can be expended before the switch to anaerobic energy production.
43. Consider the graph of lactate production as a function of effort shown in Problem 42. For an athlete racing at her lactate threshold or just below it, the RQ value will be 1. With the finish line in sight, our runner ups her pace so that she is now also processing glucose to lactic acid in addition to processing glucose aerobically; that is, she begins running above her lactate threshold. The lactic acid released into the blood will ionize

The increase in H+ will alter the blood buffer system, leading to the formation of carbonic acid
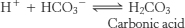
The carbonic acid will dissociate into water and carbon dioxide
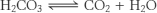
This carbon dioxide will be superimposed on the carbon dioxide generated by the combustion of glucose aerobically, leading to an RQ greated than 1.
44. The increase in ATP resulting from the processing of glucose in the β cells closes a potassium channel. The closing of the potassium channel alters the voltage across the cell membrane, which leads to an opening of a calcium channel. The influx of calcium causes the insulin-