Chapter 7
Chapter 7
1. The whale swims long distances between breaths. A high concentration of myoglobin in the whale muscle maintains a ready supply of oxygen for the muscle between breathing episodes.
2. (a) 2.96 × 10−11 g
(b) 2.74 × 108 molecules
(c) No. There would be 3.17 × 108 hemoglobin molecules in a red cell if they were packed in a cubic crystalline array. Hence, the actual packing density is about 84% of the maximum possible.
3. 2.65 g (or 4.75 × 10−2 mol) of Fe
4. (a) In human beings, 1.44 × 10−2 g (4.49 × 10−4 mol) of O2 per kilogram of muscle. In sperm whale, 0.144 g (4.49 × 10−3 mol) of O2 per kilogram.
(b) 128
5. The pKa is (a) lowered; (b) raised; and (c) raised.
6. Deoxy Hb A contains a complementary site, and so it can add on to a fiber of deoxy Hb S. The fiber cannot then grow further, because the terminal deoxy Hb A molecule lacks a sticky patch.
7. 62.7% oxygen-
8. Myoglobin does not exhibit a Bohr effect. The interactions responsible for mediating the Bohr effect in hemoglobin are dependent upon a tetrameric structure. Myoglobin is a monomer.
9. A higher concentration of BPG would shift the oxygen-
10. (a) The transfusion would increase the number of red blood cells, which increases the oxygen-
(b) BPG stabilizes the T-
11. Oxygen binding appears to cause the copper ions and their associated histidine ligands to move closer to one another, thereby also moving the helices to which the histidines are attached (in similar fashion to the conformational change in hemoglobin).
12. The modified hemoglobin should not show cooperativity. Although the imidazole in solution will bind to the heme iron (in place of histidine) and will facilitate oxygen binding, the imidazole lacks the crucial connection to the particular α helix that must move so as to transmit the change in conformation.
13. Inositol pentaphosphate (part c) is highly anionic, much like 2,3-
14.
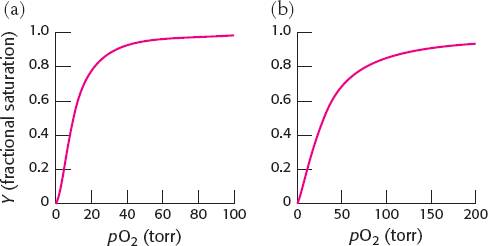
15. Release of acid will lower the pH. A lower pH promotes oxygen dissociation in the tissues. However, the enhanced release of oxygen in the tissues will increase the concentration of deoxy-
16. (a) Y = 0.5 when pO2 = 10 torr. The plot of Y versus pO2 appears to indicate little or no cooperativity.
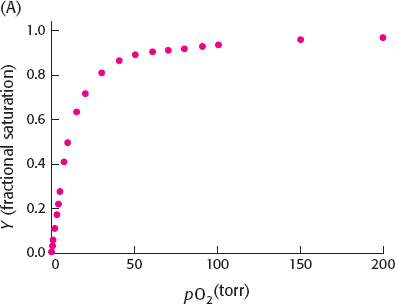
(b) The Hill plot shows slight cooperativity with n = 1.3 in the central region.
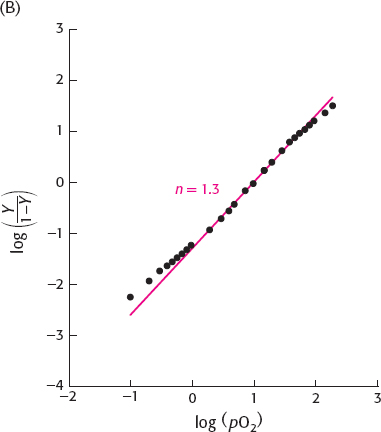
(c) Deoxy dimers of lamprey hemoglobin could have lower affinity for oxygen than do the monomers. If the binding of the first oxygen atom to a dimer causes dissociation of the dimer to give two monomers, then the process would be cooperative. In this mechanism, oxygen binding to each monomer would be easier than binding the first oxygen atom to a deoxy dimer.
17. (a) 2; (b) 4; (c) 2; (d) 1.
18. The electrostatic interactions between BPG and hemoglobin would be weakened by competition with water molecules. The T state would not be stabilized.
19. Fetal hemoglobin contains two α chains and two γ chains. The increase in levels of the γ chain provides an alternative to the mutated β chain in sickle-