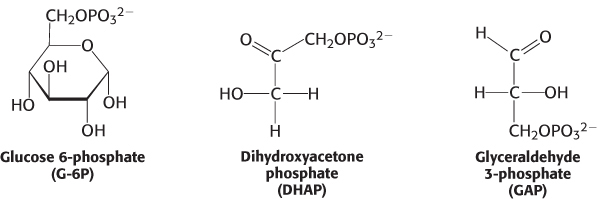
11.1
Monosaccharides Are the Simplest Carbohydrates
Carbohydrates are carbon-based molecules that are rich in hydroxyl groups. Indeed, the empirical formula for many carbohydrates is (CH2O)n—literally, a carbon hydrate. Simple carbohydrates are called monosaccharides. These simple sugars serve not only as fuel molecules but also as fundamental constituents of living systems. For instance, DNA has a backbone consisting of alternating phosphoryl groups and deoxyribose, a cyclic five-carbon sugar.
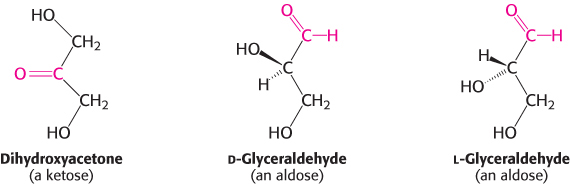
Monosaccharides are aldehydes or ketones that have two or more hydroxyl groups. The smallest monosaccharides, composed of three carbon atoms, are dihydroxyacetone and d- and l-glyceraldehyde.
Dihydroxyacetone is called a ketose because it contains a keto group (in red above), whereas glyceraldehyde is called an aldose because it contains an aldehyde group (also in red). They are referred to as trioses (tri- for three, referring to the three carbon atoms that they contain). Similarly, simple monosaccharides with four, five, six, and seven carbon atoms are called tetroses, pentoses, hexoses, and heptoses, respectively. Perhaps the monosaccharides of which we are most aware are the hexoses, such as glucose and fructose. Glucose is an essential energy source for virtually all forms of life. Fructose is commonly used as a sweetener that is converted into glucose derivatives inside the cell.
Carbohydrates can exist in a dazzling variety of isomeric forms (Figure 11.1). Dihydroxyacetone and glyceraldehyde are constitutional isomers because they have identical molecular formulas but differ in how the atoms are ordered. Stereoisomers are isomers that differ in spatial arrangement. Recall from the discussion of amino acids that stereoisomers are designated as having either d or l configuration. Glyceraldehyde has a single asymmetric carbon atom and, thus, there are two stereoisomers of this sugar: d-glyceraldehyde and L-glyceraldehyde. These molecules are a type of stereoisomer called enantiomers, which are mirror images of each other. Most vertebrate monosaccharides have the d configuration. According to convention, the d and l isomers are determined by the configuration of the asymmetric carbon atom farthest from the aldehyde or keto group. Dihydroxyacetone is the only monosaccharide without at least one asymmetric carbon atom.
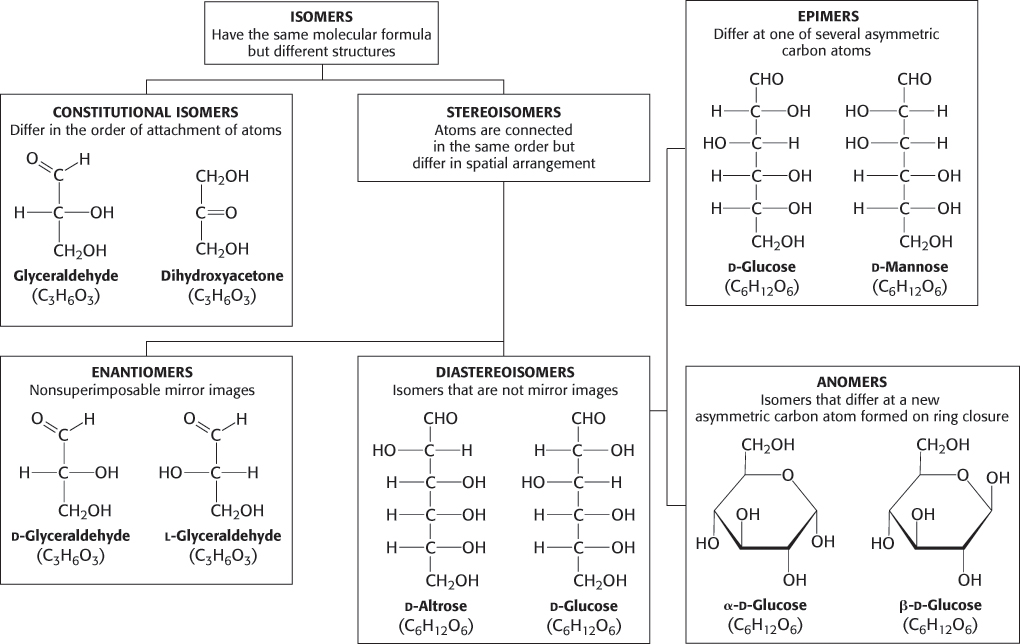
FIGURE 11.1Isomeric forms of carbohydrates.
Monosaccharides made up of more than three carbon atoms have multiple asymmetric carbons, and so they can exist not only as enantiomers but also as diastereoisomers, isomers that are not mirror images of each other. The number of possible stereoisomers equals 2n, where n is the number of asymmetric carbon atoms. Thus, a six-carbon aldose with 4 asymmetric carbon atoms can exist as 16 possible diastereoisomers, of which glucose is one such isomer.
Figure 11.2 shows the common sugars that we will see most frequently in our study of biochemistry. d-Ribose, the carbohydrate component of RNA, is a five-carbon aldose, as is deoxyribose, the monosaccharide component of deoxynucleotides. d-Glucose, d-mannose, and d-galactose are abundant six-carbon aldoses. Note that d-glucose and d-mannose differ in configuration only at C-2, the carbon atom in the second position. Sugars that are diastereoisomers differing in configuration at only a single asymmetric center are epimers. Thus, d-glucose and d-mannose are epimeric at C-2; d-glucose and d-galactose are epimeric at C-4.
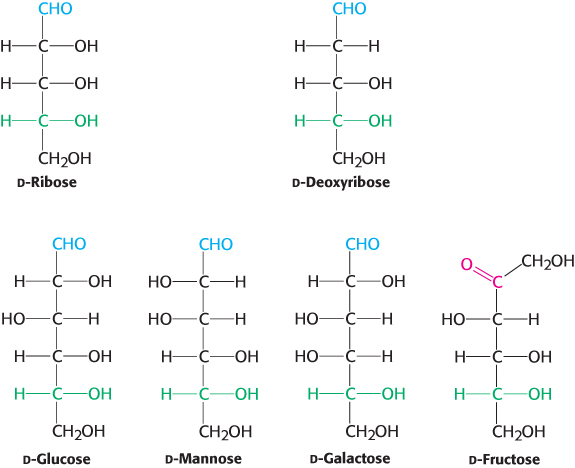
FIGURE 11.2Common monosaccharides. Aldoses contain an aldehyde (shown in blue), whereas ketoses, such as fructose, contain a ketose (shown in red). The asymmetric carbon atom farthest from the aldehyde or ketone (shown in green) designates the structures as being in the d configuration.
Note that ketoses have one less asymmetric center than aldoses with the same number of carbon atoms. d-Fructose is the most abundant ketohexose.
Many common sugars exist in cyclic forms
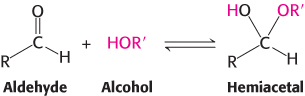
The predominant forms of ribose, glucose, fructose, and many other sugars in solution, as is the case inside the cell, are not open chains. Rather, the open-chain forms of these sugars cyclize into rings. The chemical basis for ring formation is that an aldehyde can react with an alcohol to form a hemiacetal.
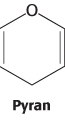
For an aldohexose such as glucose, a single molecule provides both the aldehyde and the alcohol: the C-1 aldehyde in the open-chain form of glucose reacts with the C-5 hydroxyl group to form an intramolecular hemiacetal (Figure 11.3). The resulting cyclic hemiacetal, a six-membered ring, is called pyranose because of its similarity to pyran.
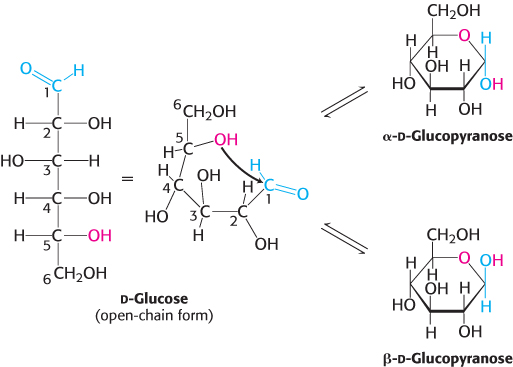
FIGURE 11.3Pyranose formation. The open-chain form of glucose cyclizes when the C-5 hydroxyl group attacks the oxygen atom of the C-1 aldehyde group to form an intramolecular hemiacetal. Two anomeric forms, designated α and β, can result.
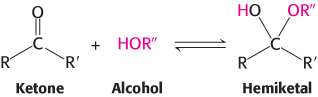
Similarly, a ketone can react with an alcohol to form a hemiketal.
The C-2 keto group in the open-chain form of a ketohexose, such as fructose, can form an intramolecular hemiketal by reacting with either the C-6 hydroxyl group to form a six-membered cyclic hemiketal or the C-5 hydroxyl group to form a five-membered cyclic hemiketal (Figure 11.4). The five-membered ring is called a furanose because of its similarity to furan.
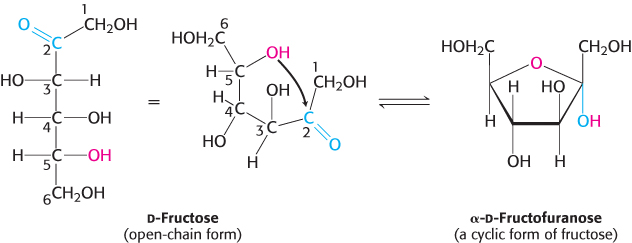
FIGURE 11.4Furanose formation. The open-chain form of fructose cyclizes to a five-membered ring when the C-5 hydroxyl group attacks the C-2 ketone to form an intramolecular hemiketal. Two anomers are possible, but only the α anomer is shown.
The depictions of glucopyranose (glucose) and fructofuranose (fructose) shown in Figures 11.3 and 11.4 are Haworth projections. In such projections, the carbon atoms in the ring are not written out. The approximate plane of the ring is perpendicular to the plane of the paper, with the heavy line on the ring projecting toward the reader.
We have seen that carbohydrates can contain many asymmetric carbon atoms. An additional asymmetric center is created when a cyclic hemiacetal is formed, generating yet another diastereoisomeric form of sugars called anomers. In glucose, C-1 (the carbonyl carbon atom in the open-chain form) becomes an asymmetric center. Thus, two ring structures can be formed: α-d-glucopyranose and β-d-glucopyranose (Figure 11.3). For d sugars drawn as Haworth projections in the standard orientation as shown in Figure 11.3, the designation α means that the hydroxyl group attached to C-1 is on the opposite side of the ring as C-6; β means that the hydroxyl group is on the same side of the ring as C-6. The C-1 carbon atom is called the anomeric carbon atom. An equilibrium mixture of glucose contains approximately one-third α anomer, two-thirds β anomer, and < 1% of the open-chain form.
The furanose-ring form of fructose also has anomeric forms, in which α and β refer to the hydroxyl groups attached to C-2, the anomeric carbon atom (Figure 11.4). Fructose forms both pyranose and furanose rings. The pyranose form predominates in fructose that is free in solution, and the furanose form predominates in many fructose derivatives (Figure 11.5).
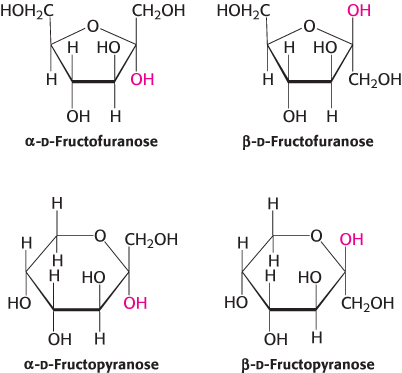
FIGURE 11.5Ring structures of fructose. Fructose can form both five-membered furanose (top) and six-membered pyranose (bottom) rings. In each case, both α and β anomers are possible.
β-d-Fructopyranose, found in honey, is one of the sweetest chemicals known. The β-d-fructofuranose form is not nearly as sweet. Heating converts β-fructopyranose into the β-fructofuranose form, reducing the sweetness of the solution. For this reason, corn syrup with a high concentration of fructose in the β-d-pyranose form is used as a sweetener in cold, but not hot, drinks. Figure 11.6 shows the common sugars discussed previously in their ring forms.
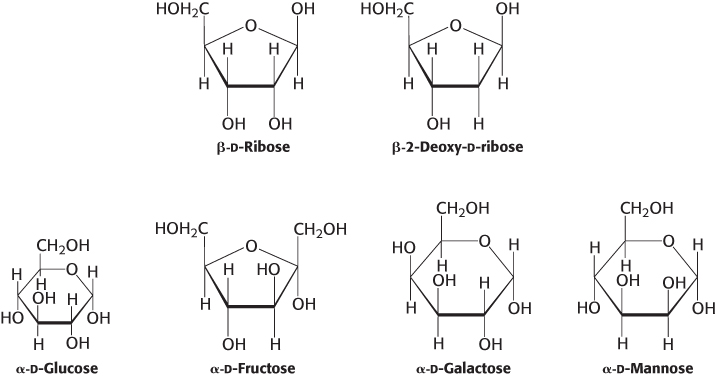
FIGURE 11.6Common monosaccharides in their ring forms.
Pyranose and furanose rings can assume different conformations
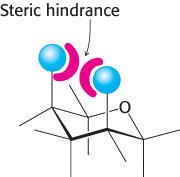
The six-membered pyranose ring is not planar because of the tetrahedral geometry of its saturated carbon atoms. Instead, pyranose rings adopt two classes of conformations, termed chair and boat because of the resemblance to these objects (Figure 11.7). In the chair form, the substituents on the ring carbon atoms have two orientations: axial and equatorial. Axial bonds are nearly perpendicular to the average plane of the ring, whereas equatorial bonds are nearly parallel to this plane. Axial substituents sterically hinder each other if they emerge on the same side of the ring (e.g., 1,3-diaxial groups). In contrast, equatorial substituents are less crowded. The chair form of β-d-glucopyranose predominates because all axial positions are occupied by hydrogen atoms. The bulkier —OH and —CH2OH groups emerge at the less-hindered periphery. The boat form of glucose is disfavored because it is quite sterically hindered.
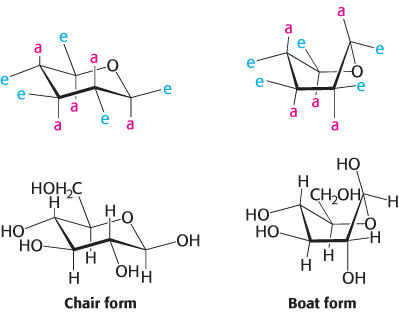
FIGURE 11.7Chair and boat forms of β-d-glucose. The chair form is more stable because hydrogen atoms occupy the axial positions, resulting in less steric hindrance. Abbreviations: a, axial; e, equatorial.
Furanose rings, like pyranose rings, are not planar. They can be puckered so that four atoms are nearly coplanar and the fifth is about 0.5 Å away from this plane (Figure 11.8). This conformation is called an envelope form because the structure resembles an opened envelope with the back flap raised. In the ribose moiety of most biomolecules, either C-2 or C-3 is out of the plane on the same side as C-5. These conformations are called C-2-endo and C-3-endo, respectively.
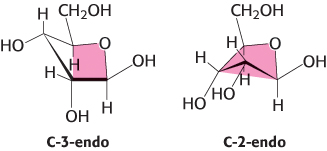
FIGURE 11.8Envelope conformations of β-d-ribose. The C-3-endo and C-2-endo forms of β-d-ribose are shown. The color indicates the four atoms that lie approximately in a plane.
Glucose is a reducing sugar
Because the α and β isomers of glucose are in an equilibrium that passes through the open-chain form, glucose has some of the chemical properties of free aldehydes, such as the ability to react with oxidizing agents. For example, glucose can react with cupric ion (Cu2+), reducing it to cuprous ion (Cu+), while being oxidized to gluconic acid.
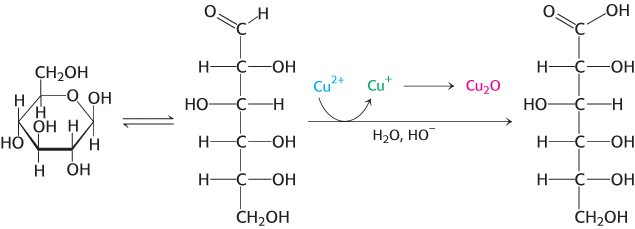
 Solutions of cupric ion (known as Fehling’s solution) provide a simple test for the presence of sugars such as glucose. Sugars that react are called reducing sugars; those that do not are called nonreducing sugars. Reducing sugars can often nonspecifically react with a free amino group to form a stable covalent bond. For instance, as a reducing sugar, glucose reacts with hemoglobin to form glycosylated hemoglobin (hemoglobin A1c). Monitoring changes in the amount of glycosylated hemoglobin is an especially useful means of assessing the effectiveness of treatments for diabetes mellitus, a condition characterized by high levels of blood glucose (Section 27.3). Because the glycosylated hemoglobin remains in circulation, the amount of the modified hemoglobin corresponds to the long-term regulation—over several months—of glucose levels. In nondiabetic individuals, less than 6% of the hemoglobin is glycosylated, whereas, in uncontrolled diabetics, almost 10% of the hemoglobin is glycosylated. Although the glycosylation of hemoglobin has no effect on oxygen binding and is thus benign, similar reducing reactions are often detrimental because the glycosylations alter the normal biochemical function of the modified proteins. These modifcations, known as advanced glycation end products (AGE), have been implicated in aging, arteriosclerosis, and diabetes, as well as other pathological conditions.
Solutions of cupric ion (known as Fehling’s solution) provide a simple test for the presence of sugars such as glucose. Sugars that react are called reducing sugars; those that do not are called nonreducing sugars. Reducing sugars can often nonspecifically react with a free amino group to form a stable covalent bond. For instance, as a reducing sugar, glucose reacts with hemoglobin to form glycosylated hemoglobin (hemoglobin A1c). Monitoring changes in the amount of glycosylated hemoglobin is an especially useful means of assessing the effectiveness of treatments for diabetes mellitus, a condition characterized by high levels of blood glucose (Section 27.3). Because the glycosylated hemoglobin remains in circulation, the amount of the modified hemoglobin corresponds to the long-term regulation—over several months—of glucose levels. In nondiabetic individuals, less than 6% of the hemoglobin is glycosylated, whereas, in uncontrolled diabetics, almost 10% of the hemoglobin is glycosylated. Although the glycosylation of hemoglobin has no effect on oxygen binding and is thus benign, similar reducing reactions are often detrimental because the glycosylations alter the normal biochemical function of the modified proteins. These modifcations, known as advanced glycation end products (AGE), have been implicated in aging, arteriosclerosis, and diabetes, as well as other pathological conditions.
Monosaccharides are joined to alcohols and amines through glycosidic bonds
The biochemical properties of monosaccharides can by modified by reaction with other molecules. These modifications increase the biochemical versatility of carbohydrates, enabling them to serve as signal molecules or facilitating their metabolism. Three common reactants are alcohols, amines, and phosphates. A bond formed between the anomeric carbon atom of a carbohydrate and the oxygen atom of an alcohol is called a glycosidic bond—specifically, an O-glycosidic bond. O-Glycosidic bonds are prominent when carbohydrates are linked together to form long polymers and when they are attached to proteins (Figure 11.9). In addition, the anomeric carbon atom of
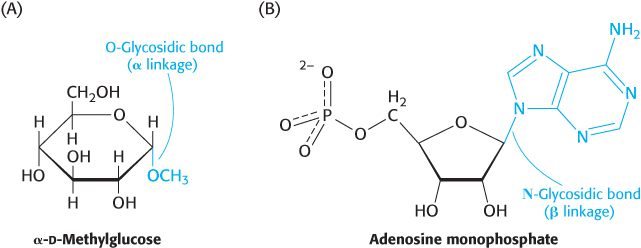
FIGURE 11.9O- and N-glycosidic linkages.(A) An O-glycosidic bond links glucose to a methyl group in α-d-methylglucose. (B) An N-glycosidic bond joins ribose to the base adenine in adenosine monophosphate.
a sugar can be linked to the nitrogen atom of an amine to form an N-glycosidic bond, such as when nitrogenous bases are attached to ribose units to form nucleosides. Carbohydrates can also be modifed by the attachment of functional groups to carbons other than the anomeric carbon (Figure 11.10).
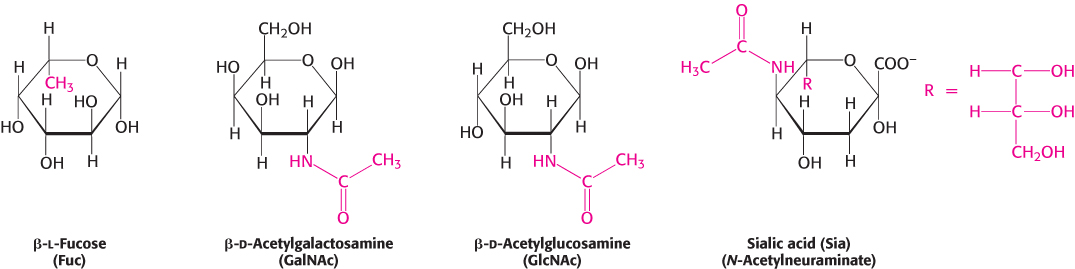
FIGURE 11.10Modified monosaccharides. Carbohydrates can be modified by the addition of substituents (shown in red) other than hydroxyl groups. Such modified carbohydrates are often expressed on cell surfaces.
Phosphorylated sugars are key intermediates in energy generation and biosyntheses
One sugar modification deserves special note because of its prominence in metabolism. The addition of phosphoryl groups is a common modification of sugars. For instance, the first step in the breakdown of glucose to obtain energy is its conversion into glucose 6-phosphate. Several subsequent intermediates in this metabolic pathway, such as dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, are phosphorylated sugars.
Phosphorylation makes sugars anionic; the negative charge not only prevents these sugars from spontaneously leaving the cell by crossing lipid-bilayer membranes, but also prevents them from interacting with transporters of the unmodified sugar. Moreover, phosphorylation creates reactive intermediates that will more readily undergo metabolism. For example, a multiply phosphorylated derivative of ribose plays key roles in the biosyntheses of purine and pyrimidine nucleotides (Chapter 25).









 Solutions of cupric ion (known as Fehling’s solution) provide a simple test for the presence of sugars such as glucose. Sugars that react are called reducing sugars; those that do not are called nonreducing sugars. Reducing sugars can often nonspecifically react with a free amino group to form a stable covalent bond. For instance, as a reducing sugar, glucose reacts with hemoglobin to form glycosylated hemoglobin (hemoglobin A1c). Monitoring changes in the amount of glycosylated hemoglobin is an especially useful means of assessing the effectiveness of treatments for diabetes mellitus, a condition characterized by high levels of blood glucose (Section 27.3). Because the glycosylated hemoglobin remains in circulation, the amount of the modified hemoglobin corresponds to the long-
Solutions of cupric ion (known as Fehling’s solution) provide a simple test for the presence of sugars such as glucose. Sugars that react are called reducing sugars; those that do not are called nonreducing sugars. Reducing sugars can often nonspecifically react with a free amino group to form a stable covalent bond. For instance, as a reducing sugar, glucose reacts with hemoglobin to form glycosylated hemoglobin (hemoglobin A1c). Monitoring changes in the amount of glycosylated hemoglobin is an especially useful means of assessing the effectiveness of treatments for diabetes mellitus, a condition characterized by high levels of blood glucose (Section 27.3). Because the glycosylated hemoglobin remains in circulation, the amount of the modified hemoglobin corresponds to the long-