12.1Fatty Acids Are Key Constituents of Lipids
Fatty Acids Are Key Constituents of Lipids
The hydrophobic properties of lipids are essential to their ability to form membranes. Most lipids owe their hydrophobic properties to one component, their fatty acids.
Fatty acid names are based on their parent hydrocarbons
Fatty acids are long hydrocarbon chains of various lengths and degrees of unsaturation that terminate with carboxylic acid groups. The systematic name for a fatty acid is derived from the name of its parent hydrocarbon by the substitution of oic for the final e. For example, the C18 saturated fatty acid is called octadecanoic acid because the parent hydrocarbon is octadecane. A C18 fatty acid with one double bond is called octadecenoic acid; with two double bonds, octadecadienoic acid; and with three double bonds, octadecatrienoic acid. The notation 18:0 denotes a C18 fatty acid with no double bonds, whereas 18:2 signifies that there are two double bonds. The structures of the ionized forms of two common fatty acids—
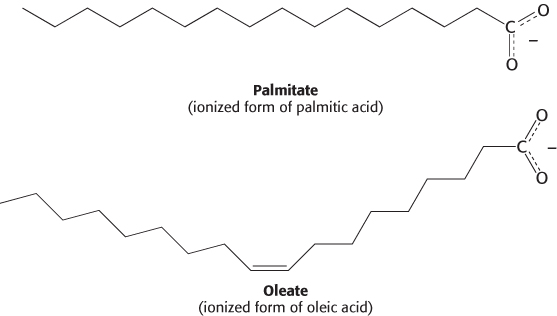
343
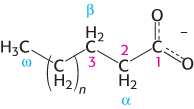
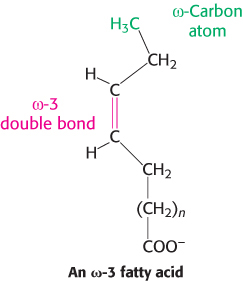
Fatty acid carbon atoms are numbered starting at the carboxyl terminus, as shown in the margin. Carbon atoms 2 and 3 are often referred to as α and β, respectively. The methyl carbon atom at the distal end of the chain is called the ω-carbon atom. The position of a double bond is represented by the symbol Δ followed by a superscript number. For example, cis-Δ9 means that there is a cis double bond between carbon atoms 9 and 10; trans-Δ2 means that there is a trans double bond between carbon atoms 2 and 3. Alternatively, the position of a double bond can be denoted by counting from the distal end, with the ω-carbon atom (the methyl carbon) as number 1. An ω-3 fatty acid, for example, has the structure shown in the margin. Fatty acids are ionized at physiological pH, and so it is appropriate to refer to them according to their carboxylate form: for example, palmitate or hexadecanoate.
Fatty acids vary in chain length and degree of unsaturation
Fatty acids in biological systems usually contain an even number of carbon atoms, typically between 14 and 24 (Table 12.1). The 16-
|
Number of carbons |
Number of double bonds |
Common name |
Systematic name |
Formula |
|---|---|---|---|---|
|
12 |
0 |
Laurate |
n-Dodecanoate |
CH3(CH2)10COO− |
|
14 |
0 |
Myristate |
n-Tetradecanoate |
CH3(CH2)12COO− |
|
16 |
0 |
Palmitate |
n-Hexadecanoate |
CH3(CH2)14COO− |
|
18 |
0 |
Stearate |
n-Octadecanoate |
CH3(CH2)16COO− |
|
20 |
0 |
Arachidate |
n-Eicosanoate |
CH3(CH2)18COO− |
|
22 |
0 |
Behenate |
n-Docosanoate |
CH3(CH2)20COO− |
|
24 |
0 |
Lignocerate |
n-Tetracosanoate |
CH3(CH2)22COO− |
|
16 |
1 |
Palmitoleate |
cis-Δ9-Hexadecenoate |
CH3(CH2)5CH=CH(CH2) 7COO− |
|
18 |
1 |
Oleate |
cis- Δ9-Octadecenoate |
CH3(CH2)7CH=CH(CH2) 7COO− |
|
18 |
2 |
Linoleate |
cis,cis- Δ9, Δ12-Octadecadienoate |
CH3(CH2)4(CH=CHCH2)2(CH)6COO− |
|
18 |
3 |
Linolenate |
all- |
CH3CH2(CH=CHCH2)3(CH2)6COO− |
|
20 |
4 |
Arachidonate |
all- |
CH3(CH2)4(CH=CHCH2)4(CH2)2COO− |
344
The properties of fatty acids and of lipids derived from them are markedly dependent on chain length and degree of saturation. Unsaturated fatty acids have lower melting points than do saturated fatty acids of the same length. For example, the melting point of stearic acid is 69.6°C, whereas that of oleic acid (which contains one cis double bond) is 13.4°C. The melting points of polyunsaturated fatty acids of the C18 series are even lower. Chain length also affects the melting point, as illustrated by the fact that the melting temperature of palmitic acid (C16) is 6.5 degrees lower than that of stearic acid (C18). Thus, short chain length and unsaturation enhance the fluidity of fatty acids and of their derivatives.