13.1The Transport of Molecules Across a Membrane May Be Active or Passive
The Transport of Molecules Across a Membrane May Be Active or Passive
We first consider some general principles of membrane transport. Two factors determine whether a molecule will cross a membrane: (1) the permeability of the molecule in a lipid bilayer and (2) the availability of an energy source.
Many molecules require protein transporters to cross membranes
As stated in Chapter 12, some molecules can pass through cell membranes because they dissolve in the lipid bilayer. Such molecules are called lipophilic molecules. The steroid hormones provide a physiological example. These cholesterol relatives can pass through a membrane, but what determines the direction in which they will move? Such molecules will pass through a membrane down their concentration gradient in a process called simple diffusion. In accord with the Second Law of Thermodynamics, molecules spontaneously move from a region of higher concentration to one of lower concentration.
Matters become more complicated when the molecule is highly polar. For example, sodium ions are present at 143 mM outside a typical cell and at 14 mM inside the cell. However, sodium does not freely enter the cell, because the charged ion cannot pass through the hydrophobic membrane interior. In some circumstances, as during a nerve impulse, sodium ions must enter the cell. How are they able to do so? Sodium ions pass through specific channels in the hydrophobic barrier formed by membrane proteins. This means of crossing the membrane is called facilitated diffusion because the diffusion across the membrane is facilitated by the channel. It is also called passive transport because the energy driving the ion movement originates from the ion gradient itself, without any contribution by the transport system. Channels, like enzymes, display substrate specificity in that they facilitate the transport of some ions, but not other, even closely related, ions.
369
How is the sodium gradient established in the first place? In this case, sodium must move, or be pumped, against a concentration gradient. Because moving the ion from a low concentration to a higher concentration results in a decrease in entropy, it requires an input of free energy. Protein transporters embedded in the membrane are capable of using an energy source to move the molecule up a concentration gradient. Because an input of energy from another source is required, this means of crossing the membrane is called active transport.
Free energy stored in concentration gradients can be quantified
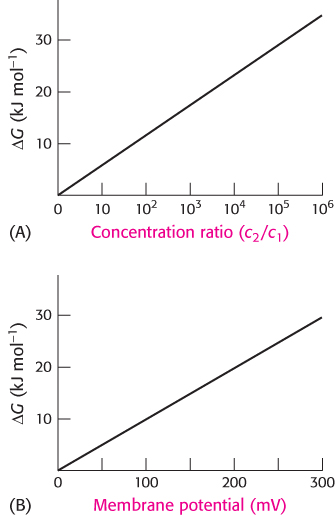
An unequal distribution of molecules is an energy-
ΔG = RT ln(c2/c1)
where R is the gas constant (8.315 × 10−3 kJ mol−1 deg−1, or 1.987 × 10−3 kcal mol−1 deg−1) and T is the temperature in kelvins. For a charged species, the unequal distribution across the membrane generates an electrical potential that also must be considered because the ions will be repelled by the like charges. The sum of the concentration and electrical terms is called the electrochemical potential or membrane potential. The free-
ΔG = RT ln(c2/c1) + ZFΔV
in which Z is the electrical charge of the transported species, ΔV is the potential in volts across the membrane, and F is the Faraday constant (96.5 kJ V−1 mol−1, or 23.1 kcal V−1 mol−1).
A transport process must be active when ΔG is positive, whereas it can be passive when ΔG is negative. For example, consider the transport of an uncharged molecule from c1 = 10−3 M to c2 = 10−1 M.
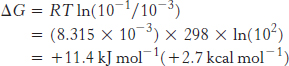
At 25°C (298 K), ΔG is +11.4 kJ mol−1 (+2.7 kcal mol−1), indicating that this transport process requires an input of free energy.
370