13.3Lactose Permease Is an Archetype of Secondary Transporters That Use One Concentration Gradient to Power the Formation of Another
Lactose Permease Is an Archetype of Secondary Transporters That Use One Concentration Gradient to Power the Formation of Another
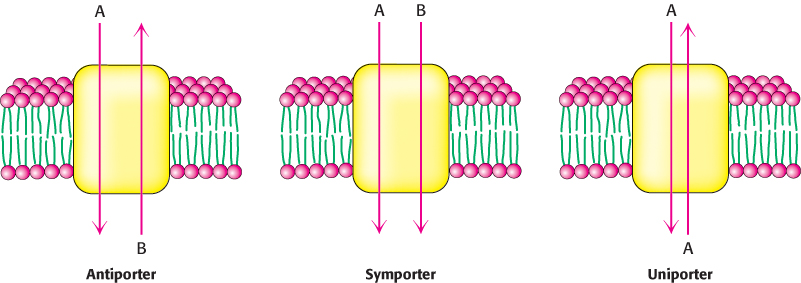
Carriers are proteins that transport ions or molecules across the membrane without hydrolysis of ATP. The mechanism of carriers involves both large conformational changes and the interaction of the protein with only a few molecules per transport cycle, limiting the maximum rate at which transport can occur. Although carriers cannot mediate primary active transport, owing to their inability to hydrolyze ATP, they can couple the thermodynamically unfavorable flow of one species of ion or molecule up a concentration gradient to the favorable flow of a different species down a concentration gradient, a process referred to as secondary active transport. Carriers that move ions or molecules “uphill” by this means are termed secondary transporters or cotransporters. These proteins can be classified as either antiporters or symporters. Antiporters couple the downhill flow of one species to the uphill flow of another in the opposite direction across the membrane; symporters use the flow of one species to drive the flow of a different species in the same direction across the membrane. Uniporters, another class of carriers, are able to transport a specific species in either direction governed only by concentrations of that species on either side of the membrane (Figure 13.10).
377
Secondary transporters are ancient molecular machines, common today in bacteria and archaea as well as in eukaryotes. For example, approximately 160 (of around 4000) proteins encoded by the E. coli genome are secondary transporters. Sequence comparison and hydropathy analysis suggest that members of the largest family have 12 transmembrane helices that appear to have arisen by duplication and fusion of a membrane protein containing 6 transmembrane helices. Included in this family is the lactose permease of E. coli. This symporter uses the H+ gradient across the E. coli membrane (outside has higher H+ concentration) generated by the oxidation of fuel molecules to drive the uptake of lactose and other sugars against a concentration gradient. This transporter has been extensively studied for many decades and is a useful archetype for this family.
The structure of lactose permease has been determined (Figure 13.11). As expected from the sequence analysis, the protein consists of two halves, each of which comprises six membrane-
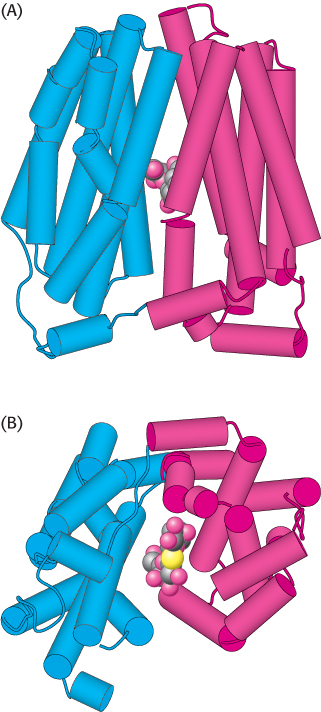
 FIGURE 13.11 Structure of lactose permease with a bound lactose analog. The amino-
FIGURE 13.11 Structure of lactose permease with a bound lactose analog. The amino-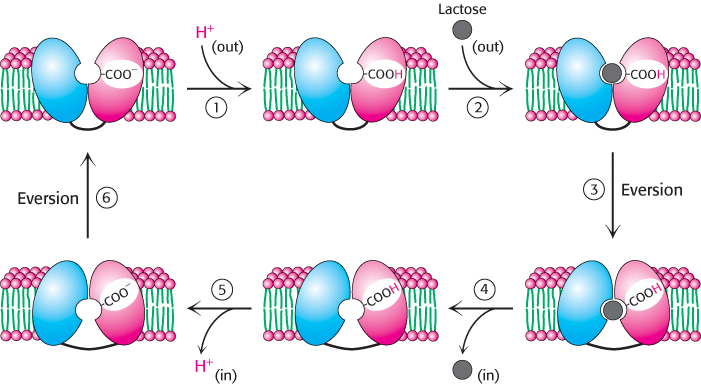
1. The cycle begins with the two halves oriented so that the opening to the binding pocket faces outside the cell, in a conformation different from that observed in the structures solved to date. A proton from outside the cell binds to a residue in the permease, quite possibly Glu 269.
2. In the protonated form, the permease binds lactose from outside the cell.
3. The structure everts to the form observed in the crystal structure (Figure 13.11).
4. The permease releases lactose to the inside of the cell.
5. The permease releases a proton to the inside of the cell.
6. The permease everts to complete the cycle.
The site of protonation likely changes in the course of this cycle.
It is believed that this eversion mechanism applies to all classes of secondary transporters, which resemble the lactose permease in overall architecture.
378