14.2 Insulin Signaling: Phosphorylation Cascades Are Central to Many Signal-Transduction Processes
The signaling pathways that we have examined so far have activated a protein kinase as a downstream component of the pathway. We now turn to a class of signal-transduction pathways that are initiated by receptors that include protein kinases as part of their structures. The activation of these protein kinases sets in motion other processes that ultimately modify the effectors of these pathways.
An example is the signal-transduction pathway initiated by insulin, the hormone released in response to increased blood-glucose levels after a meal. In all of its detail, this multifaceted pathway is quite complex. Hence, we will focus solely on the major branch, which leads to the mobilization of glucose transporters to the cell surface. These transporters allow the cell to take up the glucose that is plentiful in the blood stream after a meal.
The insulin receptor is a dimer that closes around a bound insulin molecule
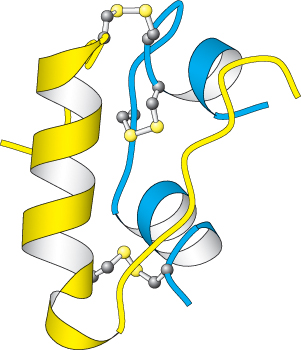
Figure 14.19:  Insulin structure. Notice that insulin consists of two chains (shown in blue and yellow) linked by two interchain disulfide bonds. The α chain (blue) also has an intrachain disulfide bond.
Insulin structure. Notice that insulin consists of two chains (shown in blue and yellow) linked by two interchain disulfide bonds. The α chain (blue) also has an intrachain disulfide bond.
[Drawn from 1B2F.pdb.]
Insulin is a peptide hormone that consists of two chains that are linked by three disulfide bonds (Figure 14.19). Its receptor has a quite different structure from that of the β-AR. The insulin receptor is a dimer of two identical units. Each unit consists of one α chain and one β chain linked to one another by a single disulfide bond (Figure 14.20). Each α subunit lies completely outside the cell, whereas each β subunit lies primarily inside the cell, spanning the membrane with a single transmembrane segment. The two α subunits move together to form a binding site for a single insulin molecule, a surprising occurrence because two different surfaces on the insulin molecule must interact with the two identical insulin-receptor chains. The moving together of the dimeric units in the presence of an insulin molecule sets the signaling pathway in motion. The closing up of an oligomeric receptor or the oligomerization of monomeric receptors around a bound ligand is a strategy used by many receptors to initiate a signal, particularly by those containing a protein kinase.
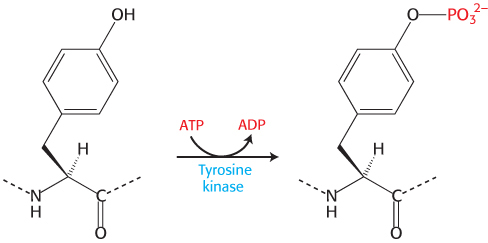
Each β subunit consists primarily of a protein kinase domain, homologous to protein kinase A. However, this kinase differs from protein kinase A in two important ways. First, the insulin-receptor kinase is a tyrosine kinase; that is, it catalyzes the transfer of a phosphoryl group from ATP to the hydroxyl group of tyrosine, rather than serine or threonine.
Because this tyrosine kinase is a component of the receptor itself, the insulin receptor is referred to as a receptor tyrosine kinase. Second, the insulin receptor kinase is in an inactive conformation when the domain is not covalently modified. The kinase is rendered inactive by the position of an unstructured loop (called the activation loop) that lies in the center of the structure.
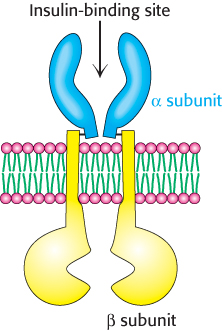
Figure 14.20: The insulin receptor. The receptor consists of two units, each of which consists of an α subunit and a β subunit linked by a disulfide bond. Two α subunits, which lie outside the cell, come together to form a binding site for insulin. Each β subunit lies primarily inside the cell and includes a protein kinase domain.
Insulin binding results in the cross-phosphorylation and activation of the insulin receptor
When the two α subunits move together to surround an insulin molecule, the two protein kinase domains on the inside of the cell also are drawn together. It is important to note that as they come together, the flexible activation loop of one kinase subunit is able to fit into the active site of the other kinase subunit within the dimer. With the two β subunits forced together, the kinase domains catalyze the addition of phosphoryl groups from ATP to tyrosine residues in the activation loops. When these tyrosine residues are phosphorylated, a striking conformational change takes place (Figure 14.21). The rearrangement of the activation loop converts the kinase into an active conformation. Thus, insulin binding on the outside of the cell results in the activation of a membrane-associated kinase within the cell.
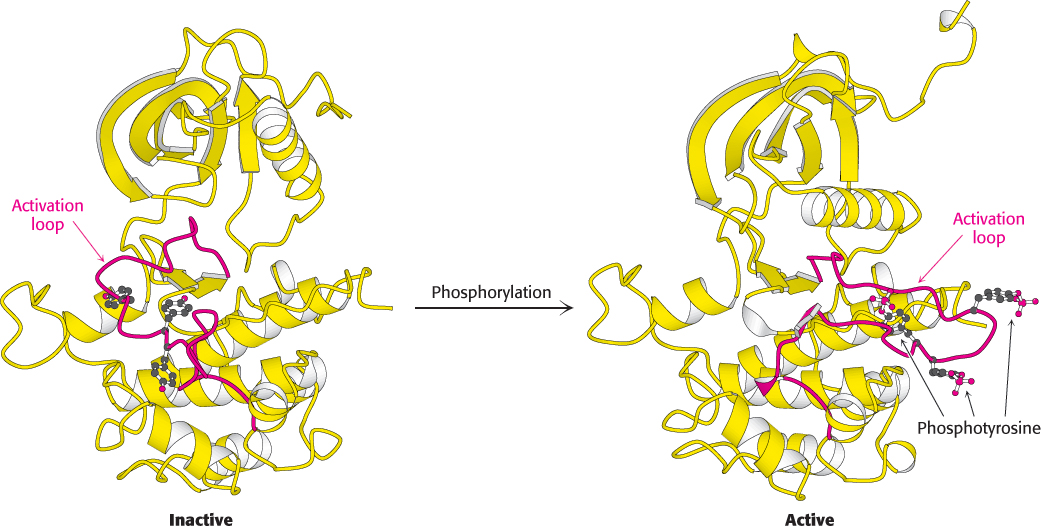
Figure 14.21:  Activation of the insulin receptor by phosphorylation. The activation loop is shown in red in this model of the protein kinase domain of the β subunit of the insulin receptor. The unphosphorylated structure on the left is not catalytically active. Notice that, when three tyrosine residues in the activation loop are phosphorylated, the activation loop swings across the structure and the kinase structure adopts a more compact conformation. This conformation is catalytically active.
Activation of the insulin receptor by phosphorylation. The activation loop is shown in red in this model of the protein kinase domain of the β subunit of the insulin receptor. The unphosphorylated structure on the left is not catalytically active. Notice that, when three tyrosine residues in the activation loop are phosphorylated, the activation loop swings across the structure and the kinase structure adopts a more compact conformation. This conformation is catalytically active.
[Drawn from 1IRK.pdb and 1IR3.pdb.]
The activated insulin-receptor kinase initiates a kinase cascade
On phosphorylation, the insulin-receptor tyrosine kinase is activated. Because the two units of the receptor are held in close proximity to one another, additional sites within the receptor also are phosphorylated. These phosphorylated sites act as docking sites for other substrates, including a class of molecules referred to as insulin-receptor substrates (IRS) Figure 14.22). IRS-1 and IRS-2 are two homologous proteins with a common modular structure (Figure 14.23). The amino- terminal part includes a pleckstrin homology domain, which binds phosphoinositide, and a phosphotyrosine-binding domain. These domains act together to anchor the IRS protein to the insulin receptor and the associated membrane. Each IRS protein contains four sequences that approximate the form Tyr-X-X-Met. These sequences are also substrates for the activated insulin-receptor kinase. When the tyrosine residues within these sequences are phosphorylated to become phosphotyrosine residues, IRS molecules can act as adaptor proteins: they are not enzymes but serve to tether the downstream components of this signaling pathway to the membrane.
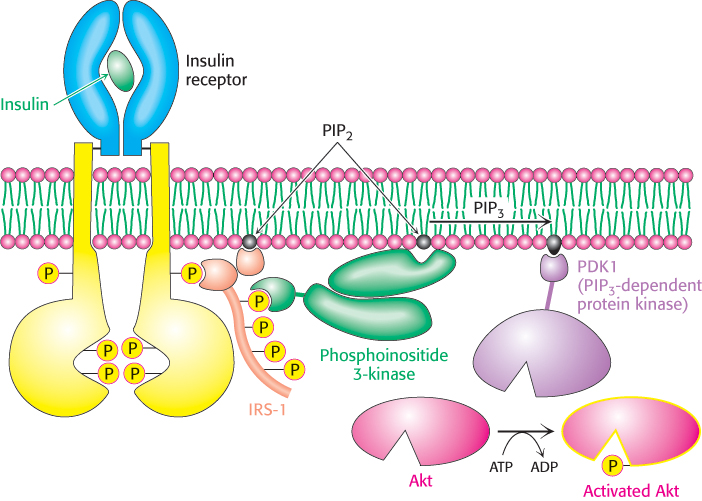
Figure 14.22: Insulin signaling. The binding of insulin results in the cross-phosphorylation and activation of the insulin receptor. Phosphorylated sites on the receptor act as binding sites for insulin-receptor substrates such as IRS-1. The lipid kinase phosphoinositide 3-kinase binds to phosphorylated sites on IRS-1 through its regulatory domain, then converts PIP2 into PIP3. Binding to PIP3 activates PIP3-dependent protein kinase (PDK1), which phosphorylates and activates kinases such as Akt1. Activated Akt1 can then diffuse throughout the cell to continue the signal-transduction pathway.
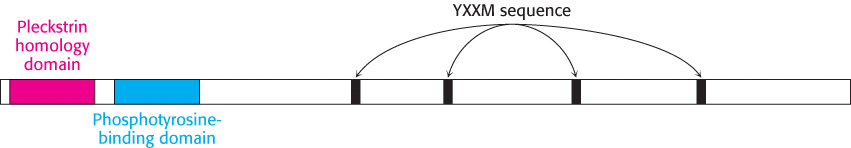
Figure 14.23: The modular structure of insulin-receptor substrates IRS-1 and IRS-2. This schematic view represents the amino acid sequence common to IRS-1 and IRS-2. Each protein contains a pleckstrin homology domain (which binds phosphoinositide lipids), a phosphotyrosine-binding domain, and four sequences that approximate Tyr-X-X-Met (YXXM). The four sequences are phosphorylated by the insulin-receptor tyrosine kinase.
Phosphotyrosine residues, such as those in the IRS proteins, are recognized most often by Src homology 2 (SH2) domains (Figure 14.24). These domains, present in many signal-transduction proteins, bind to stretches of polypeptide that contain phosphotyrosine residues. Each specific SH2 domain shows a binding preference for phosphotyrosine in a particular sequence context. Which proteins contain SH2 domains that bind to phosphotyrosine-containing sequences in the IRS proteins? The most important of them are in a class of lipid kinases, called phosphoinositide 3-kinases (PI3Ks), that add a phosphoryl group to the 3-position of inositol in phosphatidylinositol 4,5-bisphosphate (PIP2; Figure 14.25). These enzymes are heterooligomers that consist of 110- kDa catalytic subunits and 85- kDa regulatory subunits. Through SH2 domains in the regulatory subunits, these enzymes bind to the IRS proteins and are drawn to the membrane where they can phosphorylate PIP2 to form phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3, in turn, activates a protein kinase, PDK1, by virtue of a pleckstrin homology domain present in this kinase that is specific for PIP3 (Figure 14.22). The activated PDK1 phosphorylates and activates Akt, another protein kinase. Akt is not membrane anchored and moves through the cell to phosphorylate targets that include components that control the trafficking of the glucose receptor GLUT4 to the cell surface as well as enzymes that stimulate glycogen synthesis (Section 21.4).
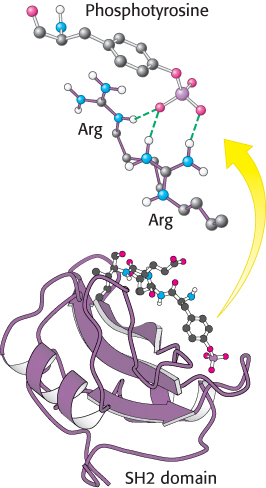
Figure 14.24:  Structure of the SH2 domain. The domain is shown bound to a phosphotyrosine-containing peptide. Notice at the top that the negatively charged phosphotyrosine residue interacts with two arginine residues that are conserved in essentially all SH2 domains.
Structure of the SH2 domain. The domain is shown bound to a phosphotyrosine-containing peptide. Notice at the top that the negatively charged phosphotyrosine residue interacts with two arginine residues that are conserved in essentially all SH2 domains.
[Drawn from 1SPS.pdb.]
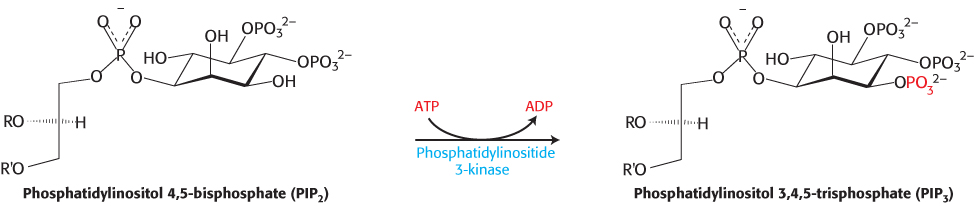
Figure 14.25: Action of a lipid kinase in insulin signaling. Phosphorylated IRS-1 and IRS-2 activate the enzyme phosphatidylinositide 3-kinase, an enzyme that converts PIP2 into PIP3.
The cascade initiated by the binding of insulin to the insulin receptor is summarized in Figure 14.26. The signal is amplified at several stages along this pathway. Because the activated insulin receptor itself is a protein kinase, each activated receptor can phosphorylate multiple IRS molecules. Activated enzymes further amplify the signal in at least two of the subsequent steps. Thus, a small increase in the concentration of circulating insulin can produce a robust intracellular response. Note that although the insulin pathway described here may seem complicated, it is substantially less elaborate than the full signaling network initiated by insulin.
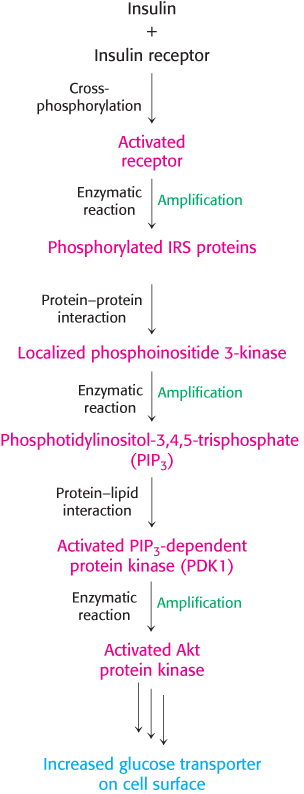
Figure 14.26: Insulin signaling pathway. Key steps in the signal-transduction pathway initiated by the binding of insulin to the insulin receptor.
Insulin signaling is terminated by the action of phosphatases
We have seen that the activated G protein promotes its own inactivation by the release of a phosphoryl group from GTP. In contrast, proteins phosphorylated on serine, threonine, or tyrosine residues are extremely stable kinetically. Specific enzymes, called protein phosphatases, are required to hydrolyze these phosphorylated proteins and return them to their initial states. Similarly, lipid phosphatases are required to remove phosphoryl groups from inositol lipids that had been activated by lipid kinases. In insulin signaling, three classes of enzymes are of particular importance in shutting off the signaling pathway: (1) protein tyrosine phosphatases that remove phosphoryl groups from tyrosine residues on the insulin receptor and the IRS adaptor proteins, (2) lipid phosphatases that hydrolyze PIP3 to PIP2, and (3) protein serine phosphatases that remove phosphoryl groups from activated protein kinases such as Akt. Many of these phosphatases are activated or recruited as part of the response to insulin. Thus, the binding of the initial signal sets the stage for the eventual termination of the response.

 Insulin structure. Notice that insulin consists of two chains (shown in blue and yellow) linked by two interchain disulfide bonds. The α chain (blue) also has an intrachain disulfide bond.
Insulin structure. Notice that insulin consists of two chains (shown in blue and yellow) linked by two interchain disulfide bonds. The α chain (blue) also has an intrachain disulfide bond.


 Activation of the insulin receptor by phosphorylation. The activation loop is shown in red in this model of the protein kinase domain of the β subunit of the insulin receptor. The unphosphorylated structure on the left is not catalytically active. Notice that, when three tyrosine residues in the activation loop are phosphorylated, the activation loop swings across the structure and the kinase structure adopts a more compact conformation. This conformation is catalytically active.
Activation of the insulin receptor by phosphorylation. The activation loop is shown in red in this model of the protein kinase domain of the β subunit of the insulin receptor. The unphosphorylated structure on the left is not catalytically active. Notice that, when three tyrosine residues in the activation loop are phosphorylated, the activation loop swings across the structure and the kinase structure adopts a more compact conformation. This conformation is catalytically active.



 Structure of the SH2 domain. The domain is shown bound to a phosphotyrosine-
Structure of the SH2 domain. The domain is shown bound to a phosphotyrosine-
