PROBLEMS
PROBLEMS
Question 14.1
Active mutants. Some protein kinases are inactive unless they are phosphorylated on key serine or threonine residues. In some cases, active enzymes can be generated by mutating these serine or threonine residues to aspartate. Explain.
Question 14.2
In the pocket. SH2 domains bind phosphotyrosine residues in deep pockets on their surfaces. Would you expect SH2 domains to bind phosphoserine and phosphothreonine with high affinity? Why or why not?
Question 14.3
On–
Question 14.4
Viva la différence. Why is the fact that a monomeric hormone binds simultaneously to two identical receptor molecules, thus promoting the formation of a dimer of the receptor, considered remarkable?
Question 14.5
Antibodies mimicking hormones. Antibodies have two identical antigen-
Question 14.6
Facile exchange. A mutated form of the α subunit of the heterotrimeric G protein has been identified; this form readily exchanges nucleotides even in the absence of an activated receptor. What would be the effect on a signaling pathway containing the mutated α subunit?
Question 14.7
Making connections. Suppose that you were investigating a newly discovered growth-
Question 14.8
Diffusion rates. Usually, rates of diffusion vary inversely with molecular weights; so smaller molecules diffuse faster than do larger ones. In cells, however, calcium ion diffuses more slowly than does cAMP. Propose a possible explanation.
Question 14.9
Negativity abounds. Fura-
Question 14.10
Awash with glucose. Glucose is mobilized for ATP generation in muscle in response to epinephrine, which activates Gαs. Cyclic AMP phosphodiesterase is an enzyme that converts cAMP into AMP. How would inhibitors of cAMP phosphodiesterase affect glucose mobilization in muscle?
Question 14.11
Getting it started. The insulin receptor, on dimerization, cross-
Question 14.12
Many defects. Considerable effort has been directed toward determining the genes in which sequence variation contributes to the development of type 2 diabetes. Approximately 800 genes have been implicated. Propose an explanation for this observation.
Question 14.13
Growth-
Question 14.14
Receptor truncation. You prepare a cell line that overexpresses a mutant form of EGFR in which the entire intracellular region of the receptor has been deleted. Predict the effect of overexpression of this construct on EGF signaling in this cell line.
421
Question 14.15
Hybrid. Suppose that, through genetic manipulations, a chimeric receptor is produced that consists of the extracellular domain of the insulin receptor and the transmembrane and intracellular domains of the EGF receptor. Cells expressing this receptor are exposed to insulin, and the level of phosphorylation of the chimeric receptor is examined. What would you expect to observe and why? What would you expect to observe if these cells were exposed to EGF?
Question 14.16
Total amplification. Suppose that each β-adrenergic receptor bound to epinephrine converts 100 molecules of Gαs into their GTP forms and that each molecule of activated adenylate cyclase produces 1000 molecules of cAMP per second. With the assumption of a full response, how many molecules of cAMP will be produced in 1 s after the formation of a single complex between epinephrine and the β-adrenergic receptor?
Chapter Integration Problems
Question 14.17
Nerve-
Question 14.18
Redundancy. Because of the high degree of genetic variability in tumors, typically no single anticancer therapy is universally effective for all patients, even within a given tumor type. Hence, it is often desirable to inhibit a particular pathway at more than one point in the signaling cascade. In addition to the EGFR-
Mechanism Problems
Question 14.19
Distant relatives. The structure of adenylate cyclase is similar to the structures of some types of DNA polymerases, suggesting that these enzymes derived from a common ancestor. Compare the reactions catalyzed by these two enzymes. In what ways are they similar?
Question 14.20
Kinase inhibitors as drugs. Functional and structural analysis indicates that Gleevec is an ATP-
Data Interpretation Problems
Question 14.21
Establishing specificity. You wish to determine the hormone-
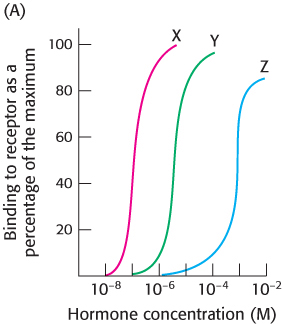
What concentrations of each hormone yield 50% maximal binding?
Which hormone shows the highest binding affinity for the receptor?
You next wish to determine whether the hormone–
receptor complex stimulates the adenylate cyclase cascade. To do so, you measure adenylate cyclase activity as a function of hormone concentration, as shown in graph B. 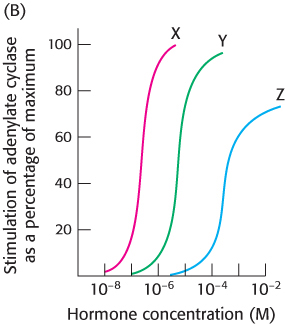
What is the relation between the binding affinity of the hormone–
receptor complex and the ability of the hormone to enhance adenylate cyclase activity? What can you conclude about the mechanism of action of the hormone– receptor complex? Suggest experiments that would determine whether a Gαs protein is a component of the signal-
transduction pathway.
422
Question 14.22
Binding issues. A scientist wishes to determine the number of receptors specific for a ligand X, which he has in both radioactive and nonradioactive form. In one experiment, he adds increasing amounts of radioactive X and measures how much of it is bound to the cells. The result is shown as total activity in the following graph. Next, he performs the same experiment, except that he includes a several hundredfold excess of nonradioactive X. This result is shown as nonspecific binding. The difference between the two curves is the specific binding.
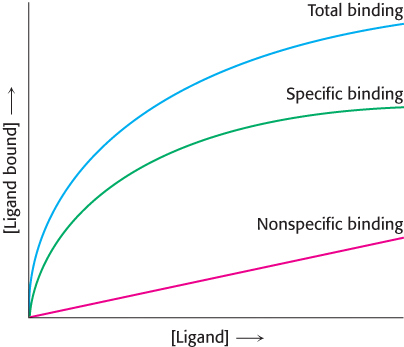
Why is the total binding not an accurate representation of the number of receptors on the cell surface?
What is the purpose of performing the experiment in the presence of excess nonradioactive ligand?
What is the significance of the fact that specific binding attains a plateau?
Question 14.23
Counting receptors. With the use of experiments such as those described in Problems 21 and 22, the number of receptors in the cell membrane can be calculated. Suppose that the specific activity of the ligand is 1012 cpm per millimole and that the maximal specific binding is 104 cpm per milligram of membrane protein. There are 1010 cells per milligram of membrane protein. Assume that one ligand binds per receptor. Calculate the number of receptor molecules present per cell.