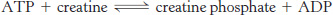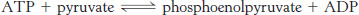PROBLEMS
Question 15.1
Complex patterns. What is meant by intermediary metabolism?
Question 15.2
Opposites. Differentiate between anabolism and catabolism.
Question 15.3
Graffiti. While walking to biochemistry class with a friend, you see the following graffiti spray painted on the wall of the science building: “When a system is in equilibrium, the Gibbs free energy is maximum.” You are disgusted, not only at the vandalism, but at the ignorance of the vandal. Your friend asks you to explain.
Question 15.4
Why bother to eat? What are the three primary uses for cellular energy?
Question 15.5
Match ’em.
|
1. Cellular energy currency _____ |
a. NAD+ |
|
2. Anabolic electron carrier _____ |
b. Coenzyme A |
|
3. Phototroph _____ |
c. Precursor to coenzymes |
|
4. Catabolic electron carrier reaction _____ |
d. Yields energy |
|
5. Oxidation- |
e. Requires energy |
|
6. Activated carrier of two carbon fragments _____ |
f. ATP |
|
7. Vitamin _____ |
g. Transfers electrons |
|
8. Anabolism _____ |
h. NADP+ |
|
9. Amphibolic reaction _____ |
i. Converts light energy to chemical energy |
|
10. Catabolism _____ |
j. Used in anabolism and catabolism |
Question 15.6
Charges. In vivo, ATP is usually bound to magnesium or manganese ions. Why is this the case?
Question 15.7
Energy to burn. What factors account for the high phosphoryl-
Question 15.8
Back in time. Account for the fact that ATP, and not another nucleoside triphosphate, is the cellular energy currency.
Question 15.9
Currency issues. Why does it make good sense to have a single nucleotide, ATP, function as the cellular energy currency?
Question 15.10
Environmental conditions. The standard free energy of hydrolysis for ATP is −30.5 kJ mol−1 (−7.3 kcal mol−1).

What conditions might be changed to alter the free energy of hydrolysis?
Question 15.11
Brute force? Metabolic pathways frequently contain reactions with positive standard free energy values, yet the reactions still take place. How is this possible?
447
Question 15.12
Energy flow. What is the direction of each of the following reactions when the reactants are initially present in equimolar amounts? Use the data given in Table 15.1.
Question 15.13
A proper inference. What information do the ΔG°′ data given in Table 15.1 provide about the relative rates of hydrolysis of pyrophosphate and acetyl phosphate?
Question 15.14
A potent donor. Consider the following reaction:
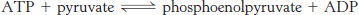
Calculate ΔG°′ and
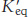 at 25°C for this reaction by using the data given in Table 15.1.
at 25°C for this reaction by using the data given in Table 15.1.What is the equilibrium ratio of pyruvate to phosphoenolpyruvate if the ratio of ATP to ADP is 10?
Question 15.15
Isomeric equilibrium. Using the information in Table 15.1, calculate ΔG°′ for the isomerization of glucose 6-
Question 15.16
Activated acetate. The formation of acetyl CoA from acetate is an ATP-

Calculate ΔG°′ for this reaction by using data given in this chapter.
The PPi formed in the preceding reaction is rapidly hydrolyzed in vivo because of the ubiquity of inorganic pyrophosphatase. The ΔG°′ for the hydrolysis of PPi is −19.2 kJ mol−1 (−4.6 kcal mol−1). Calculate the ΔG°′ for the overall reaction, including pyrophosphate hydrolysis. What effect does the hydrolysis of PPi have on the formation of acetyl CoA?
Question 15.17
Acid strength. The pK of an acid is a measure of its proton-
Derive a relation between ΔG°′ and pK.
What is the ΔG°′ for the ionization of acetic acid, which has a pK of 4.8?
Question 15.18
Raison d’être. The muscles of some invertebrates are rich in arginine phosphate (phosphoarginine). Propose a function for this amino acid derivative.
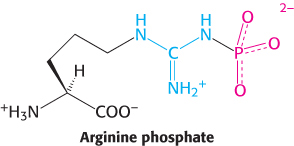
Question 15.19
Recurring motif. What is the structural feature common to ATP, FAD, NAD+, and CoA?
Question 15.20
Ergogenic help or hindrance? Creatine is a popular, but untested, dietary supplement.
What is the biochemical rationale for the use of creatine?
What type of exercise would most benefit from creatine supplementation?
Question 15.21
Standard conditions versus real life 1.The enzyme aldolase catalyzes the following reaction in the glycolytic pathway:
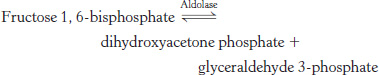
The ΔG°′ for the reaction is +23.8 kJ mol−1 (+5.7 kcal mol−1), whereas the ΔG in the cell is −1.3 kJ mol−1 (−0.3 kcal mol−1). Calculate the ratio of reactants to products under equilibrium and intracellular conditions. Using your results, explain how the reaction can be endergonic under standard conditions and exergonic under intracellular conditions.
Question 15.22
Standard conditions versus real life 2. In Section 15.2, we showed that a reaction,  , with a ΔG°′ = +16.7 kJ mol−1 (+4.0 kcal mol−1) has an
, with a ΔG°′ = +16.7 kJ mol−1 (+4.0 kcal mol−1) has an 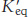 of 1.15 × 10−3. The
of 1.15 × 10−3. The 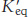 is increased to 2.67 × 102 if the reaction is coupled to ATP hydrolysis under standard conditions. The ATP-
is increased to 2.67 × 102 if the reaction is coupled to ATP hydrolysis under standard conditions. The ATP-
Question 15.23
Not all alike. The concentrations of ATP, ADP, and Pi differ with cell type. Consequently, the release of free energy with the hydrolysis of ATP will vary with cell type. Using the following table, calculate the ΔG for the hydrolysis of ATP in liver, muscle, and brain cells. In which cell type is the free energy of ATP hydrolysis most negative?
|
|
ATP (mM) |
ADP (mM) |
Pi (mM) |
|---|---|---|---|
|
Liver |
3.5 |
1.8 |
5.0 |
|
Muscle |
8.0 |
0.9 |
8.0 |
|
Brain |
2.6 |
0.7 |
2.7 |
Question 15.24
Oxidation issues. Examine the pairs of molecules and identify the more-
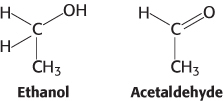
448
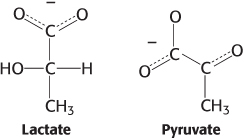
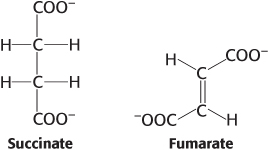
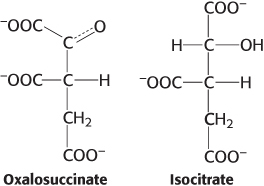
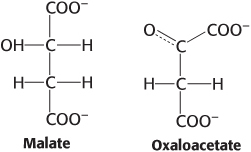
Question 15.25
Running downhill. Glycolysis is a series of 10 linked reactions that convert one molecule of glucose into two molecules of pyruvate with the concomitant synthesis of two molecules of ATP (Chapter 16). The ΔG°′ for this set of reactions is −35.6 kJ mol−1 (−8.5 kcal mol−1), whereas the ΔG is −90 kJ mol−1 (−22 kcal mol−1). Explain why the free-
Question 15.26
Outsourcing. Outsourcing, a common business practice, is contracting with another business to perform a particular function. Higher organisms were the original outsourcers, frequently depending on lower organisms to perform key biochemical functions. Give an example from this chapter of biochemical outsourcing.
Question 15.27
Breakdown products. Digestion is the first stage in the extraction of energy from food, but no useful energy is acquired during this stage. Why is digestion considered a stage in energy extraction?
Question 15.28
High-
Question 15.29
Less reverberation. Thioesters, common in biochemistry, are more unstable (energy-
Question 15.30
Classifying reactions. What are the six common types of reactions seen in biochemistry?
Question 15.31
Staying in control. What are the three principal means of controlling metabolic reactions?
Chapter Integration Problems
Question 15.32
Kinetic versus thermodynamic. The reaction of NADH with oxygen to produce NAD+ and H2O is very exergonic, yet the reaction of NADH and oxygen takes place very slowly. Why does a thermodynamically favorable reaction not occur rapidly?
Question 15.33
Activated sulfate. Fibrinogen contains tyrosine-
Data Interpretation Problem
Question 15.34
Opposites attract. The following graph shows how the ΔG for the hydrolysis of ATP varies as a function of the Mg2+ concentration (pMg = −log[Mg2+]).
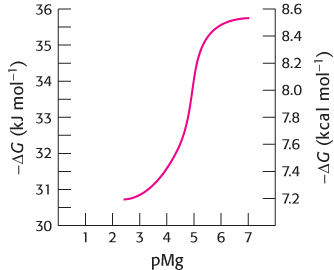
How does decreasing [Mg2+] affect the ΔG of hydrolysis for ATP?
Explain this effect.