18.1Eukaryotic Oxidative Phosphorylation Takes Place in Mitochondria
Eukaryotic Oxidative Phosphorylation Takes Place in Mitochondria
Recall that a biochemical role of the citric acid cycle, which takes place in the mitochondrial matrix, is the generation of high-
Mitochondria are bounded by a double membrane
Electron microscopic studies revealed that mitochondria have two membrane systems: an outer membrane and an extensive, highly folded inner membrane. The inner membrane is folded into a series of internal ridges called cristae. Hence, there are two compartments in mitochondria: (1) the intermembrane space between the outer and the inner membranes and (2) the matrix, which is bounded by the inner membrane (Figure 18.2). The mitochondrial matrix is the site of most of the reactions of the citric acid cycle and fatty acid oxidation. In contrast, oxidative phosphorylation takes place in the inner mitochondrial membrane. The increase in surface area of the inner mitochondrial membrane provided by the cristae creates more sites for oxidative phosphorylation than would be the case with a simple, unfolded membrane. Humans contain an estimated 14,000 m2 of inner mitochondrial membrane, which is the approximate equivalent of three football fields in the United States.
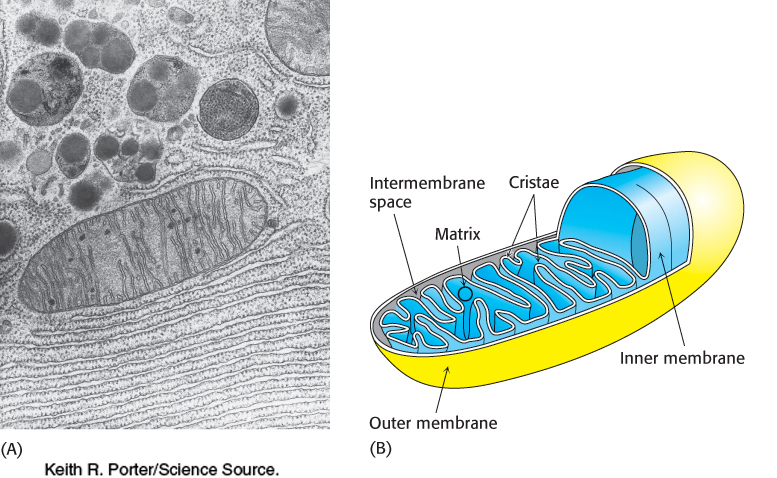
The outer membrane is quite permeable to most small molecules and ions because it contains mitochondrial porin, a 30-
525
In prokaryotes, the electron-
Mitochondria are the result of an endosymbiotic event
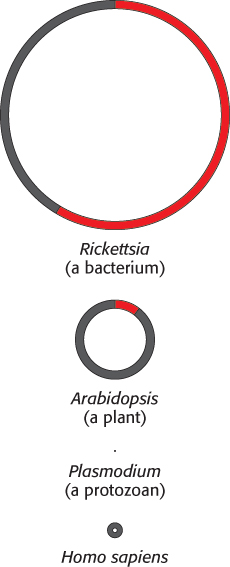
 Mitochondria are semiautonomous organelles that live in an endosymbiotic relation with the host cell. These organelles contain their own DNA, which encodes a variety of different proteins and RNAs. Mitochondrial DNA is usually portrayed as being circular, but recent research suggests that the mitochondrial DNA of many organisms may be linear. The genomes of mitochondria range broadly in size across species. The mitochondrial genome of the protist Plasmodium falciparum consists of fewer than 6000 base pairs (bp), whereas those of some land plants comprise more than 200,000 bp (Figure 18.3). Human mitochondrial DNA comprises 16,569 bp and encodes 13 respiratory-
Mitochondria are semiautonomous organelles that live in an endosymbiotic relation with the host cell. These organelles contain their own DNA, which encodes a variety of different proteins and RNAs. Mitochondrial DNA is usually portrayed as being circular, but recent research suggests that the mitochondrial DNA of many organisms may be linear. The genomes of mitochondria range broadly in size across species. The mitochondrial genome of the protist Plasmodium falciparum consists of fewer than 6000 base pairs (bp), whereas those of some land plants comprise more than 200,000 bp (Figure 18.3). Human mitochondrial DNA comprises 16,569 bp and encodes 13 respiratory-
An endosymbiotic event is thought to have occurred whereby a free-
526
The evidence that modern mitochondria result from a single event comes from examination of the most bacteria-
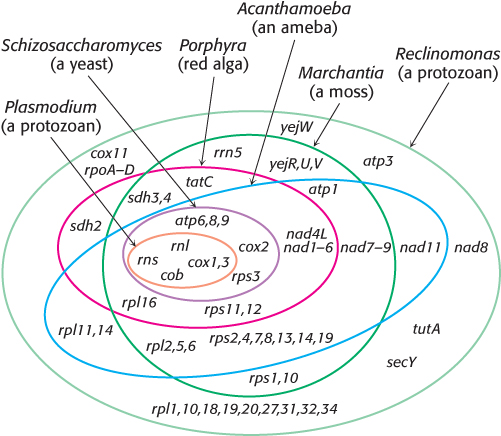
Note that transient engulfment of prokaryotic cells by larger cells is not uncommon in the microbial world. In regard to mitochondria, such a transient relationship it incapable of independent living, and the host cell became dependent on the ATP generated by its tenant.