19.6The Ability to Convert Light into Chemical Energy Is Ancient
The Ability to Convert Light into Chemical Energy Is Ancient
 The ability to convert light energy into chemical energy is a tremendous evolutionary advantage. Geological evidence suggests that oxygenic photosynthesis became important approximately 2 billion years ago. Anoxygenic photosynthetic systems arose much earlier in the 3.5-
The ability to convert light energy into chemical energy is a tremendous evolutionary advantage. Geological evidence suggests that oxygenic photosynthesis became important approximately 2 billion years ago. Anoxygenic photosynthetic systems arose much earlier in the 3.5-
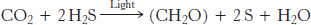
|
Bacteria |
Photosynthetic electron donor |
O2 use |
|---|---|---|
|
Green sulfur |
H2, H2S, S |
Anoxygenic |
|
Green nonsulfur |
Variety of amino acids and organic acids |
Anoxygenic |
|
Purple sulfur |
H2, H2S, S |
Anoxygenic |
|
Purple nonsulfur |
Usually organic molecules |
Anoxygenic |
|
Cyanobacteria |
H2O |
Oxygenic |
585
Nonetheless, photosynthesis did not evolve immediately at the origin of life. No photosynthetic organisms have been discovered in the domain of Archaea, implying that photosynthesis evolved in the domain of Bacteria after Archaea and Bacteria diverged from a common ancestor. All domains of life do have electron-
Artificial photosynthetic systems may provide clean, renewable energy
As we have learned, photosynthetic organisms use sunlight to oxidize H2O, producing O2 and protons used to power ATP synthesis and generate NADPH. Research is currently underway to try to mimic this process in order to provide clean energy. Photovoltaic cells can use light energy to oxidize water, producing O2 as well as H2. Hydrogen gas is a fuel that, upon reaction with oxygen, generates energy and only water as a waste product. Major difficulties in creating efficient photovoltaic cells are that the materials required are not durable and often not readily available. Recent work suggests that semiconductors composed of organic–