Glucose 6-phosphate dehydrogenase deficiency causes a drug-induced hemolytic anemia
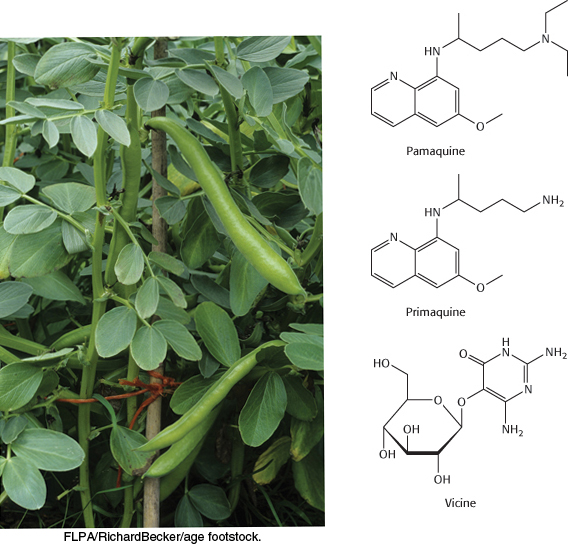
Vicia faba. The Mediterranean plant Vicia faba is a source of fava beans that contain the purine glycoside vicine.
[FLPA/RichardBecker/age footstock.]
 The importance of the pentose phosphate pathway is highlighted by some people’s anomalous responses to certain drugs. For instance, pamaquine, the first synthetic antimalarial drug introduced in 1926, was associated with the appearance of severe and mysterious ailments. Most patients tolerated the drug well, but a few developed severe symptoms within a few days after therapy was started. Their urine turned black, jaundice developed, and the hemoglobin content of the blood dropped sharply. In some cases, massive destruction of red blood cells caused death.
The importance of the pentose phosphate pathway is highlighted by some people’s anomalous responses to certain drugs. For instance, pamaquine, the first synthetic antimalarial drug introduced in 1926, was associated with the appearance of severe and mysterious ailments. Most patients tolerated the drug well, but a few developed severe symptoms within a few days after therapy was started. Their urine turned black, jaundice developed, and the hemoglobin content of the blood dropped sharply. In some cases, massive destruction of red blood cells caused death.
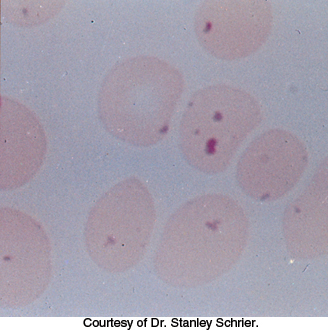
FIGURE 20.24Red blood cells with Heinz bodies. The light micrograph shows red blood cells obtained from a person deficient in glucose 6-phosphate dehydrogenase. The dark particles, called Heinz bodies, inside the cells are clumps of denatured hemoglobin that adhere to the plasma membrane and stain with basic dyes. Red blood cells in such people are highly susceptible to oxidative damage.
[Courtesy of Dr. Stanley Schrier.]
This drug-induced hemolytic anemia was shown 30 years later to be caused by a deficiency of glucose 6-phosphate dehydrogenase, the enzyme catalyzing the first step in the oxidative branch of the pentose phosphate pathway. The result is a dearth of NADPH in all cells, but this deficiency is most acute in red blood cell because they lack mitochondria and have no alternative means of generating reducing power. This defect, which is inherited on the X chromosome, is the most common disease that results from an enzyme malfunction, affecting hundreds of millions of people.
Pamaquine sensitivity is not simply a historical oddity about malaria treatment many decades ago. Primaquine, an antimalarial closely related to pamaquine, is widely used in malaria-infested regions of the world. Vicine, a purine glycoside of fava beans (which are consumed in countries surrounding the Mediterranean), also induces hemolysis. People deficient in glucose 6-phosphate dehydrogenase suffer hemolysis from eating fava beans or inhaling the pollen of the fava flowers, a response called favism. How can we explain hemolysis caused by pamaquine, primaquine, and vicine biochemically? These chemicals are oxidative agents that generate peroxides, reactive oxygen species that can damage membranes as well as other biomolecules. Peroxides are usually eliminated by the enzyme glutathione peroxidase, which uses reduced glutathione as a reducing agent.
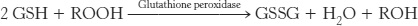
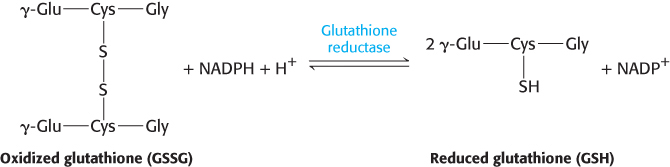
The major role of NADPH in red cells is to reduce the disulfide form of glutathione to the sulfhydryl form. The enzyme that catalyzes the regeneration of reduced glutathione is glutathione reductase.
Red blood cells with a lowered level of reduced glutathione are more susceptible to hemolysis. In the absence of glucose 6-phosphate dehydrogenase, peroxides continue to damage membranes because no NADPH is being produced to restore reduced glutathione. Thus, the answer to our question is that glucose 6-phosphate dehydrogenase is required to maintain reduced glutathione levels to protect against oxidative stress. In the absence of oxidative stress, however, the deficiency is quite benign. The sensitivity to oxidative agents of people having this dehydrogenase deficiency also clearly demonstrates that atypical reactions to drugs may have a genetic basis.
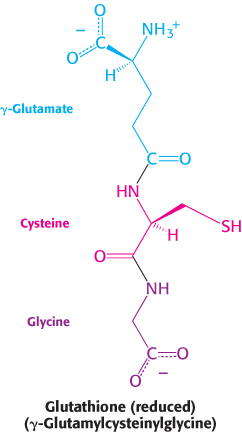
Reduced glutathione is also essential for maintaining the normal structure of red blood cells by maintaining the structure of hemoglobin. The reduced form of glutathione serves as a sulfhydryl buffer that keeps the residues of hemoglobin in the reduced sulfhydryl form. Without adequate levels of reduced glutathione, the hemoglobin sulfhydryl groups can no longer be maintained in the reduced form. Hemoglobin molecules then cross-link with one another to form aggregates called Heinz bodies on cell membranes (Figure 20.24). Membranes damaged by Heinz bodies and reactive oxygen species become deformed, and the cell is likely to undergo lysis.
A deficiency of glucose 6-phosphate dehydrogenase confers an evolutionary advantage in some circumstances
 The incidence of the most common form of glucose 6-phosphate dehydrogenase deficiency, characterized by a 10-fold reduction in enzymatic activity in red blood cells, is 11% among Americans of African heritage. This high frequency suggests that the deficiency may be advantageous under certain environmental conditions. Indeed, glucose 6-phosphate dehydrogenase deficiency protects against falciparum malaria. The parasites causing this disease require NADPH for growth. Moreover, infection by the parasites induces oxidative stress in the infected cell. Because the pentose phosphate pathway is compromised, the cell and parasite die from oxidative damage. Thus, glucose 6-phosphate dehydrogenase deficiency is a mechanism of protection against malaria, which accounts for its high frequency in malaria-infested regions of the world. We see here once again the interplay of heredity and environment in the production of disease.
The incidence of the most common form of glucose 6-phosphate dehydrogenase deficiency, characterized by a 10-fold reduction in enzymatic activity in red blood cells, is 11% among Americans of African heritage. This high frequency suggests that the deficiency may be advantageous under certain environmental conditions. Indeed, glucose 6-phosphate dehydrogenase deficiency protects against falciparum malaria. The parasites causing this disease require NADPH for growth. Moreover, infection by the parasites induces oxidative stress in the infected cell. Because the pentose phosphate pathway is compromised, the cell and parasite die from oxidative damage. Thus, glucose 6-phosphate dehydrogenase deficiency is a mechanism of protection against malaria, which accounts for its high frequency in malaria-infested regions of the world. We see here once again the interplay of heredity and environment in the production of disease.
The ability of glucose 6-phosphate dehydrogenase deficiency to protect against malaria does, however, create a public health conundrum. Primaquine is a commonly used and highly effective antimalarial drug. However, indiscriminate use of primaquine causes hemolysis in individuals deficient in glucose 6-phosphate dehydrogenase. A solution to this problem may be in the offing, as recent work shows that an anti-malaria vaccine may be within reach.

 The importance of the pentose phosphate pathway is highlighted by some people’s anomalous responses to certain drugs. For instance, pamaquine, the first synthetic antimalarial drug introduced in 1926, was associated with the appearance of severe and mysterious ailments. Most patients tolerated the drug well, but a few developed severe symptoms within a few days after therapy was started. Their urine turned black, jaundice developed, and the hemoglobin content of the blood dropped sharply. In some cases, massive destruction of red blood cells caused death.
The importance of the pentose phosphate pathway is highlighted by some people’s anomalous responses to certain drugs. For instance, pamaquine, the first synthetic antimalarial drug introduced in 1926, was associated with the appearance of severe and mysterious ailments. Most patients tolerated the drug well, but a few developed severe symptoms within a few days after therapy was started. Their urine turned black, jaundice developed, and the hemoglobin content of the blood dropped sharply. In some cases, massive destruction of red blood cells caused death.
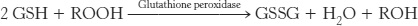
 The incidence of the most common form of glucose 6-
The incidence of the most common form of glucose 6-