PROBLEMS
PROBLEMS
Question 21.1
Step-
Question 21.2
Match ‘em. Match each term with its description.
|
(a) Glycogen phosphorylase ____ (b) Phosphorolysis ____ (c) Transferase ____ (d) α-1,6- (e) Phosphoglucomutase ____ (f) Phosphorylase kinase ____ (g) Protein kinase A ____ (h) Calmodulin ____ (i) Epinephrine ____ (j) Glucagon ____ |
1. Calcium- 2. Activates glycogen phosphorylase 3. Removal of a glucose residue by the addition of phosphate 4. Stimulates glycogen breakdown in muscle 5. Liberates a free glucose residue 6. Shifts the location of several glucose residues 7. Stimulates glycogen breakdown in the liver 8. Catalyzes phosphorolytic cleavage 9. Prepares glucose 1- 10. Phosphorylates phosphorylase kinase |
640
Question 21.3
Choice is good. Glycogen is not as reduced as fatty acids are and consequently not as energy rich. Why do animals store any energy as glycogen? Why not convert all excess fuel into fatty acids?
Question 21.4
If a little is good, a lot is better. α-Amylose is an unbranched glucose polymer. Why would this polymer not be as effective a storage form of glucose as glycogen?
Question 21.5
Telltale products. A sample of glycogen from a patient with liver disease is incubated with orthophosphate, phosphorylase, the transferase, and the debranching enzyme (α-1,6-
Question 21.6
Dare to be different. Compare the allosteric regulation of phosphorylase in the liver and in muscle, and explain the significance of the difference.
Question 21.7
A thumb on the balance. The reaction catalyzed by phosphorylase is readily reversible in vitro. At pH 6.8, the equilibrium ratio of orthophosphate to glucose 1-
Question 21.8
Excessive storage. Suggest an explanation for the fact that the amount of glycogen in type I glycogen-
Question 21.9
Recouping an essential phosphoryl. The phosphoryl group on phosphoglucomutase is slowly lost by hydrolysis. Propose a mechanism that utilizes a known catalytic intermediate for restoring this essential phosphoryl group. How might this phosphoryl donor be formed?
Question 21.10
Not all absences are equal. Hers disease results from an absence of liver glycogen phosphorylase and may result in serious illness. In McArdle disease, muscle glycogen phosphorylase is absent. Although exercise is difficult for patients suffering from McArdle disease, the disease is rarely life threatening. Account for the different manifestations of the absence of glycogen phosphorylase in the two tissues. What does the existence of these two different diseases indicate about the genetic nature of the phosphorylase?
Question 21.11
Hydrophobia. Why is water excluded from the active site of phosphorylase? Predict the effect of a mutation that allows water molecules to enter.
Question 21.12
Removing all traces. In human liver extracts, the catalytic activity of glycogenin was detectable only after treatment with α-amylase, an enzyme the hydrolyzes α-1,4-
Question 21.13
Two in one. A single polypeptide chain houses the transferase and debranching enzyme. Cite a potential advantage of this arrangement.
Question 21.14
How did they do that? A strain of mice has been developed that lack the enzyme phosphorylase kinase. Yet, after strenuous exercise, the glycogen stores of a mouse of this strain are depleted. Explain how this depletion is possible.
Question 21.15
An appropriate inhibitor. What is the rationale for the inhibition of muscle glycogen phosphorylase by glucose 6-
Question 21.16
Passing along the information. Outline the signal-
Question 21.17
Slammin’ on the breaks. There must be a way to shut down glycogen breakdown quickly to prevent the wasteful depletion of glycogen after energy needs have been met. What mechanisms are employed to turn off glycogen breakdown?
Question 21.18
Diametrically opposed. Phosphorylation has opposite effects on glycogen synthesis and breakdown. What is the advantage of its having opposing effects?
Question 21.19
Feeling depleted. Glycogen depletion resulting from intense, extensive exercise can lead to exhaustion and the inability to continue exercising. Some people also experience dizziness, an inability to concentrate, and a loss of muscle control. Account for these symptoms.
Question 21.20
Everyone had a job to do. What accounts for the fact that liver phosphorylase is a glucose sensor, whereas muscle phosphorylase is not?
Question 21.21
Yin and Yang. Match the terms on the left with the descriptions on the right.
|
(a) UDP- (b) UDP- (c) Glycogen synthase ____ (d) Glycogenin ____ (e) Branching enzyme ____ (f) Glucose 6- (g) Glycogen synthase kinase ____ (h) Protein phosphatase 1 _____ (i) Insulin _____ (j) Glycogen phosphorylase a _____ |
1. Glucose 1- 2. Potent activator of glycogen synthase b. 3. Glucose sensor in the liver. 4. Activated substrate for glycogen synthesis. 5. Synthesizes α-1,4 linkages between glucose molecules. 6. Leads to the inactivation of glycogen synthase kinase. 7. Synthesizes α-1,6 linkages between glucose molecules. 8. Catalyzes the formation of glycogen synthase b. 9. Catalyzes the formation of glycogen synthase a. 10. Provides the primer for glycogen synthesis. |
641
Question 21.22
Team effort. What enzymes are required for the synthesis of a glycogen particle starting from glucose 6-
Question 21.23
Force it forward. The following reaction accounts for the synthesis of UDP-

Question 21.24
If you insist. Why does activation of the phosphorylated b form of glycogen synthase by high concentrations of glucose 6-
Question 21.25
An ATP saved is an ATP earned. The complete oxidation of glucose 6-
Question 21.26
Dual roles. Phosphoglucomutase is crucial for glycogen breakdown as well as for glycogen synthesis. Explain the role of this enzyme in each of the two processes.
Question 21.27
Working at cross-
Question 21.28
Achieving immortality. Glycogen synthase requires a primer. A primer was formerly thought to be provided when the existing glycogen granules are divided between the daughter cells produced by cell division. In other words, parts of the original glycogen molecule were simply passed from generation to generation. Would this strategy have been successful in passing glycogen stores from generation to generation? How are new glycogen molecules now known to be synthesized?
Question 21.29
Synthesis signal. How does insulin stimulate glycogen synthesis?
Mechanism Problem
Question 21.30
Family resemblance. Enzymes of the α-amylase family (problem 12) catalyze a reaction by forming a covalent intermediate to a conserved aspartate residue. Propose mechanisms for the two enzymes catalyzing steps in glycogen debranching on the basis of their potential membership in the α-amylase family.
Chapter Integration Problems
Question 21.31
Double activation. What pathway in addition to the cAMP-
Question 21.32
Carbohydrate conversion. Write a balanced equation for the formation of glycogen from galactose.
Question 21.33
Working together. What enzymes are required for the liver to release glucose into the blood when an organism is asleep and fasting?
Question 21.34
A shattering experience. Crystals of phosphorylase a grown in the presence of glucose shatter when a substrate such as glucose 1-
Question 21.35
I know I’ve seen that face before. UDP-
Question 21.36
Same symptoms, different cause. Suggest another mutation in glucose metabolism that causes symptoms similar to those of von Gierke disease.
Data Interpretation Problems
Question 21.37
An authentic replica. Experiments were performed in which serine (S) 14 of glycogen phosphorylase was replaced by glutamate (E). The Vmax of the mutant enzyme was then compared with the wild type phosphorylase in both the a and the b form.
|
Vmax μmoles of glucose 1- |
|
|---|---|
|
Wild type phosphorylase b |
25 ± 0.4 |
|
Wild type phosphorylase a |
100 ± 5 |
|
S to E mutant |
60 ± 3 |
Explain the results obtained with the mutant.
Predict the effect of substituting aspartic acid for the serine.
Question 21.38
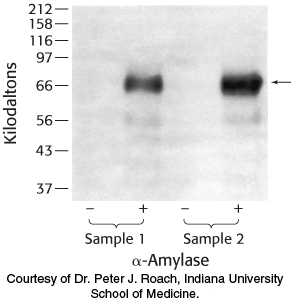
Glycogen isolation 1. The liver is a major storage site for glycogen. Purified from two samples of human liver, glycogen was either treated or not treated with α-amylase and subsequently analyzed by SDS-
Why are no proteins visible in the lanes without amylase treatment?
What is the effect of treating the samples with α-amylase? Explain the results.
List other proteins that you might expect to be associated with glycogen. Why are other proteins not visible?
642
Question 21.39
Glycogen isolation 2. The gene for glycogenin was transfected into a cell line that normally stores only small amounts of glycogen. The cells were then manipulated according to the following protocol, and glycogen was isolated and analyzed by SDS-
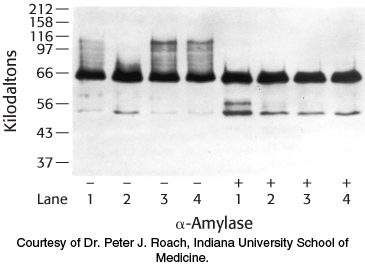
The protocol: Cells cultured in growth medium and 25 mM glucose (lane 1) were switched to medium containing no glucose for 24 hours (lane 2). Glucose-
Why did the western analysis produce a “smear”—that is, the high-
molecular- weight staining in lane 1(−)? What is the significance of the decrease in high-
molecular- weight staining in lane 2(−)? What is the significance of the difference between lanes 2(−) and 3(−)?
Suggest a plausible reason why there is essentially no difference between lanes 3(−) and 4(−).
Why are the bands at 66 kDa the same in the lanes treated with amylase, despite the fact that the cells were treated differently?