22.1Triacylglycerols Are Highly Concentrated Energy Stores
Triacylglycerols Are Highly Concentrated Energy Stores
Triacylglycerols are highly concentrated stores of metabolic energy because they are reduced and anhydrous. The yield from the complete oxidation of fatty acids is about 38 kJ g−1 (9 kcal g−1), in contrast with about 17 kJ g−1 (4 kcal g−1) for carbohydrates and proteins. The basis of this large difference in caloric yield is that fatty acids are much more reduced than carbohydrates or proteins. Furthermore, triacylglycerols are nonpolar, and so they are stored in a nearly anhydrous form, whereas much more polar carbohydrates are more highly hydrated. In fact, 1 g of dry glycogen binds about 2 g of water. Consequently, a gram of nearly anhydrous fat stores 6.75 times as much energy as a gram of hydrated glycogen, which is likely the reason that triacylglycerols rather than glycogen were selected in evolution as the major energy reservoir. Consider a typical 70-

In mammals, the major site of triacylglycerol accumulation is the cytoplasm of adipose cells (fat cells). This fuel-
The utility of triacylglycerols as an energy source is dramatically illustrated by the abilities of migratory birds, which can fly great distances without eating after having stored energy as triacylglycerols. Examples are the American golden plover and the ruby-
646
Dietary lipids are digested by pancreatic lipases
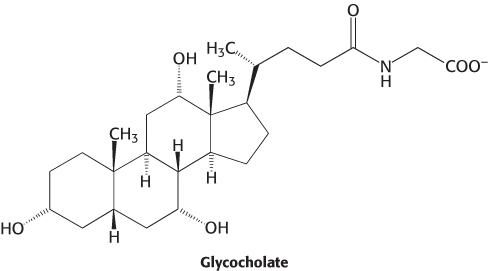
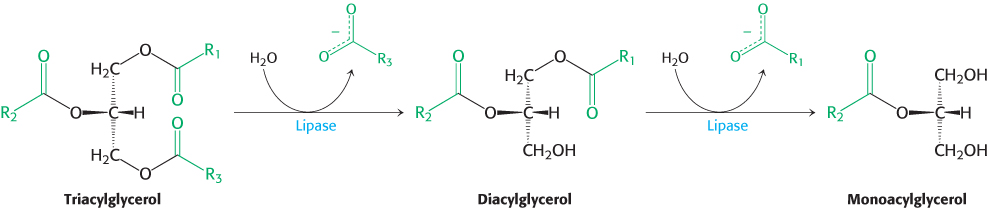
Most lipids are ingested in the form of triacylglycerols and must be degraded to fatty acids for absorption across the intestinal epithelium. Intestinal enzymes called lipases, secreted by the pancreas, degrade triacylglycerols to free fatty acids and monoacylglycerol (Figure 22.3). Lipids present a special problem because, unlike carbohydrates and proteins, these molecules are not soluble in water. They exit the stomach as an emulsion, particles with a triacylglycerol core surrounded by cholesterol and cholesterol esters. How are the lipids made accessible to the lipases, which are in aqueous solution? First, the particles are coated with bile acids (Figure 22.4), amphipathic molecules synthesized from cholesterol in the liver and secreted from the gallbladder. The ester bond s of each lipid are oriented toward the surface of the bile salt-
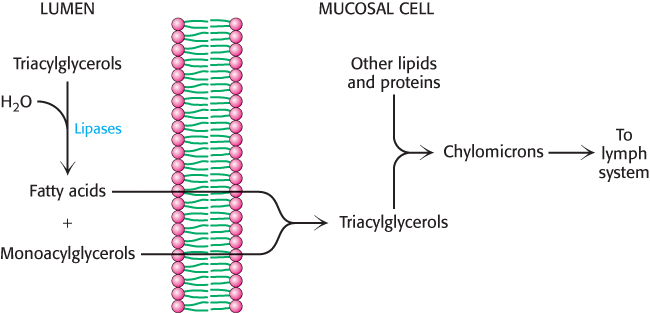
Dietary lipids are transported in chylomicrons
In the intestinal mucosal cells, the triacylglycerols are resynthesized from fatty acids and monoacylglycerols and then packaged into lipoprotein transport particles called chylomicrons, stable particles approximately 2000 Å (200 nm) in diameter (Figure 22.5). These particles are composed mainly of triacylglycerols, with apoliprotein B-
The chylomicrons are released into the lymph system and then into the blood. These particles bind to membrane-
647