PROBLEMS
PROBLEMS
Question 24.1
Out of thin air. Define nitrogen fixation. What organisms are capable of nitrogen fixation?
Question 24.2
Like Trinidad and Tobago. Match each term with its description.
|
(a) Nitrogen fixation _____ (b) Nitrogenase complex _____ (c) Glutamate _____ (d) Essential amino acids _____ (e) Nonessential amino acids _____ (f) Aminotransferase _____ (g) Pyridoxal phosphate _____ (h) Tetrahydrofolate _____ (i) S- (j) Homocysteine _____ |
1. Methylated to form methionine 2. An important methyl Donor 3. Coenzyme required by aminotransferases 4. Conversion of N2 into NH3 5. A carrier of various one- 6. Amino acids that are dietary requirements 7. Amino acids that are readily synthesized 8. Responsible for nitrogen fixation 9. Transfers amino groups between keto acids 10. A common amino group donor |
Question 24.3
Teamwork. Identify the two components of the nitrogenase complex and describe their specific tasks.
Question 24.4
The fix is in. “The mechanistic complexity of nitrogenase is necessary because nitrogen fixation is a thermodynamically unfavorable process.” True or false? Explain.
Question 24.5
Siphoning resources. Nitrogen-
Question 24.6
From few, many. What are the seven precursors of the 20 amino acids?
Question 24.7
Vital, in the truest sense. Why are certain amino acids defined as essential for human beings?
Question 24.8
From sugar to amino acid. Write a balanced equation for the synthesis of alanine from glucose.
Question 24.9
From air to blood. What are the intermediates in the flow of nitrogen from N2 to heme?
Question 24.10
Common component. What cofactor is required by all aminotransferases?
Question 24.11
Here, hold this. In this chapter, we considered three different cofactors/cosubstrates that act as carriers of one-
Question 24.12
One-
Question 24.13
Telltale tag. In the reaction catalyzed by glutamine synthetase, an oxygen atom is transferred from the side chain of glutamate to orthophosphate, as shown by the results of 18O-
Question 24.14
Telltale tag, redux. In contrast to the production of glutamine by glutamine synthetase (problem 13), the generation of asparagine from 18O-
Question 24.15
Therapeutic glycine. Isovaleric acidemia is an inherited disorder of leucine metabolism caused by a deficiency of isovaleryl CoA dehydrogenase. Many infants having this disease die in the first month of life. The administration of large amounts of glycine sometimes leads to marked clinical improvement. Propose a mechanism for the therapeutic action of glycine.
Question 24.16
Lending a hand. The atoms from tryptophan shaded below are derived from two other amino acids. Name them.
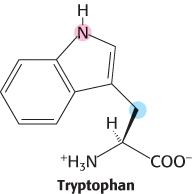
Question 24.17
Deprived bacteria. Blue-
Question 24.18
Cysteine and cystine. Most cytoplasmic proteins lack disulfide bonds, whereas extracellular proteins usually contain them. Why?
Question 24.19
Through the looking-
Question 24.20
To and fro. The synthesis of δ-aminolevulinate takes place in the mitochondrial matrix, whereas the formation of porphobilinogen takes place in the cytoplasm. Propose a reason for the mitochondrial location of the first step in heme synthesis.
741
Question 24.21
Direct synthesis. Which of the 20 amino acids can be synthesized directly from a common metabolic intermediate by a transamination reaction?
Question 24.22
Alternative route to proline. Certain species of bacteria possess an enzyme, ornithine cyclodeaminase, that can catalyze the conversion of l-ornithine into l-proline in a single catalytic cycle.
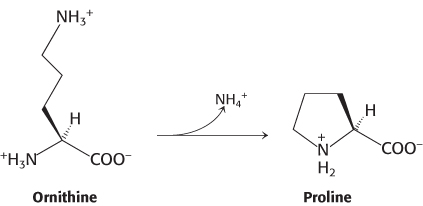
The enzyme lysine cyclodeaminase has also been identified. Predict the product of the reaction catalyzed by lysine cyclodeaminase.
Question 24.23
Lines of communication. For the following example of a branched pathway, propose a feedback inhibition scheme that would result in the production of equal amounts of Y and Z.
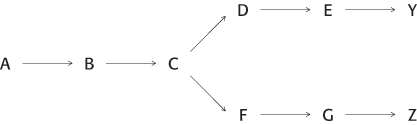
Question 24.24
Cumulative feedback inhibition. Consider the branched pathway in Problem 23. The first common step (A → B) is partly inhibited by both of the final products, each acting independently of the other. Suppose that a high level of Y alone decreased the rate of the A → B step from 100 to 60 s−1 and that a high level of Z alone decreased the rate from 100 to 40 s−1. What would the rate be in the presence of high levels of both Y and Z?
Question 24.25
Recovered activity. Free sulfhydryl groups can be alkylated with 2-
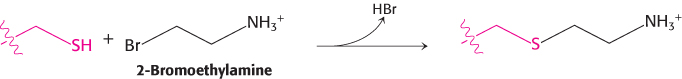
Researchers prepared a mutant form of aspartate aminotransferase in which lysine 258 was replaced by cysteine (Lys258Cys). This mutant protein has no observable catalytic activity. However, treatment of Lys258Cys with 2-
Mechanism Problems
Question 24.26
Ethylene formation. Propose a mechanism for the conversion of S-adenosylmethionine into 1-
Question 24.27
Mirror- ?
?
Question 24.28
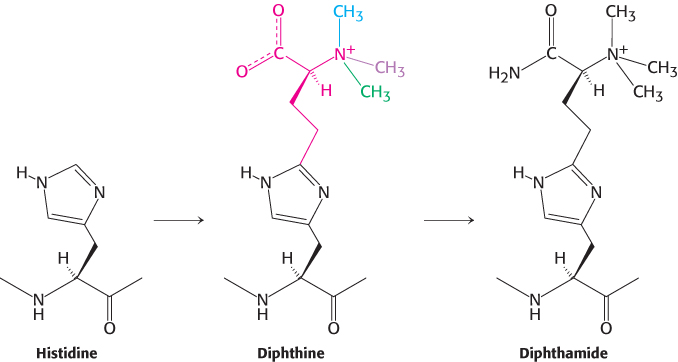
An unusual amino acid. Elongation factor-
Labeling experiments indicate that the diphthine intermediate is formed by the modification of histidine with four molecules of S-adenosylmethionine (indicated by the four colors). Propose a mechanism for the formation of diphthine.
(b) The final conversion of diphthine into diphthamide is known to be ATP dependent. Propose two possible mechanisms for the final amidation step.
742
Chapter Integration Problems
Question 24.29
Here, hold this again. In this chapter, we considered three different cofactors/cosubstrates that act as carriers of one-
Question 24.30
Connections. How might increased synthesis of aspartate and glutamate affect energy production in a cell? How would the cell respond to such an effect?
Question 24.31
Protection required. Suppose that a mutation in bacteria resulted in the diminished activity of methionine adenosyl-
Question 24.32
Heme biosynthesis. Shemin and coworkers used acetate-
Question 24.33
Comparing KM. Glutamate dehydrogenase and glutamine synthetase are present in all organisms. Most prokaryotes also contain another enzyme, glutamate synthase, which catalyzes the reductive amination of α-ketoglutarate with the use of glutamine as the nitrogen donor.
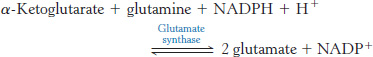
The side-
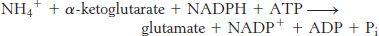
Note that this stoichiometry differs from that of the glutamate dehydrogenase reaction in that ATP is hydrolyzed. Why do prokaryotes sometimes use this more-
Chapter Integration and Data Interpretation Problem
Question 24.34
Light effects. The adjoining graph shows the concentration of several free amino acids in light-
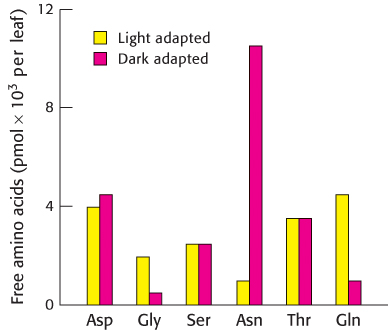
Of the amino acids shown, which are most affected by light–
dark adaptation? Suggest a plausible biochemical explanation for the difference observed.
White asparagus, a culinary delicacy, is the result of growing asparagus plants in the dark. What chemical might you think enhances the taste of white asparagus?