PROBLEMS
PROBLEMS
Question 25.1
From the beginning or extract and save and reuse. Differentiate between the de novo synthesis of nucleotides and salvage-
Question 25.2
Finding their roots 1. Identify the source of the atoms in the pyrimidine ring
Question 25.3
Finding their roots 2. Identify the source of the atoms in the purine ring.
Question 25.4
Multifaceted. List some of the biochemical roles played by nucleotides.
Question 25.5
An s instead of a t? Differentiate between a nucleoside and a nucleotide.
Question 25.6
Associate ’em.
|
(a) Excessive urate _____ (b) Lack of adenosine deaminase _____ (c) Lack of HGPRT _____ (d) Carbamoyl phosphate _____ (e) Inosinate _____ (f) Ribonucleotide reductase _____ (g) Lack of folic acid _____ (h) Glutamine phosphoribosyl transferase _____ (i) Single ring _____ (j) Bicyclic ring _____ (k) Precursor to CTP _____ |
1. Spina bifida 2. Precursor to both ATP and GTP 3. Purine 4. Deoxynucleotide synthesis 5. UTP 6. Lesch- 7. Immunodeficiency 8. Pyrimidine 9. Gout 10. First step in pyrimidine synthesis 11. Committed step in purine synthesis |
Question 25.7
Safe passage. What is substrate channeling? How does it affect enzyme efficiency?
Question 25.8
Activated ribose phosphate. Write a balanced equation for the synthesis of PRPP from glucose through the oxidative branch of the pentose phosphate pathway.
Question 25.9
Making a pyrimidine. Write a balanced equation for the synthesis of orotate from glutamine, CO2, and aspartate.
Question 25.10
Identifying the donor. What is the activated reactant in the biosynthesis of each of the following compounds?
Phosphoribosylamine
Carbamoylaspartate
Orotidylate (from orotate)
Phosphoribosylanthranilate
Question 25.11
Inhibiting purine biosynthesis. Amidotransferases are inhibited by the antibiotic azaserine (O-diazoacetyl-
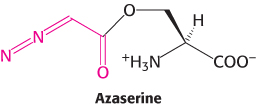
Which intermediates in purine biosynthesis would accumulate in cells treated with azaserine?
Question 25.12
The price of methylation. Write a balanced equation for the synthesis of TMP from dUMP that is coupled to the conversion of serine into glycine.
Question 25.13
Sulfa action. Bacterial growth is inhibited by sulfanilamide and related sulfa drugs, and there is a concomitant accumulation of 5-
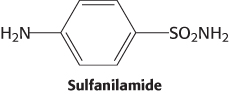
Propose a mechanism for the inhibitory effect of sulfanilamide.
Question 25.14
HAT medium. Mutant cells unable to synthesize nucleotides by salvage pathways are very useful tools in molecular and cell biology. Suppose that cell A lacks thymidine kinase, the enzyme catalyzing the phosphorylation of thymidine to thymidylate, and that cell B lacks hypoxanthine-
Cell A and cell B do not proliferate in a HAT medium containing hypoxanthine, aminopterin or amethopterin (methotrexate), and thymine. However, cell C, formed by the fusion of cells A and B, grows in this medium. Why?
Suppose that you want to introduce foreign genes into cell A. Devise a simple means of distinguishing between cells that have taken up foreign DNA and those that have not.
Question 25.15
Vitamin requirement. What is the role of folate in pyrimidine synthesis and what is the consequence of a folate deficiency during development?
Question 25.16
Bringing equilibrium. What is the reciprocal substrate relation in the synthesis of ATP and GTP?
Question 25.17
Find the label. Suppose that cells are grown on amino acids that have all been labeled at the α carbons with 13C. Identify the atoms in cytosine and guanine that will be labeled with 13C.
Question 25.18
Need a map? Describe the path for the synthesis of TTP from UTP.
Question 25.19
Adjunct therapy. Allopurinol is sometimes given to patients with acute leukemia who are being treated with anticancer drugs. Why is allopurinol used?
Question 25.20
A hobbled enzyme. Both side-
765
Question 25.21
Needed supplies. Why are cancer cells especially sensitive to inhibitors of TMP synthesis?
Question 25.22
Alternative routes. Orotic aciduria results when one of the enzyme activities of UMP synthetase is absent. This syndrome is characterized by large amounts of orotic acid in the blood and urine, megaloblastic anemia (characterized by large, immature, and dysfunctional red blood cells) and retarded growth. Suggest a possible treatment for this condition.
Question 25.23
Correcting deficiencies. Suppose that a person is found who is deficient in an enzyme required for IMP synthesis. How might this person be treated?
Question 25.24
Labeled nitrogen. Purine biosynthesis is allowed to take place in the presence of [15N]aspartate, and the newly synthesized GTP and ATP are isolated. What positions are labeled in the two nucleotides?
Question 25.25
On the trail of carbons. Tissue culture cells were incubated with glutamine labeled with 15N in the amide group. Subsequently, IMP was isolated and found to contain some 15N. Which atoms in IMP were labeled?
Question 25.26
Mechanism of action. What is the biochemical basis of allopurinol treatment for gout?
Question 25.27
Changed inhibitor. Xanthine oxidase treated with allopurinol results in the formation of a new compound that is an extremely potent inhibitor of the enzyme. Propose a structure for this compound.
Question 25.28
Calculate the ATP footprint. How many molecules of ATP are required to synthesize one molecule of CTP from scratch?
Question 25.29
Blockages. What intermediate in purine synthesis will accumulate if a strain of bacteria is lacking each of the following biochemicals?
Aspartate
Tetrahydrofolate
Glycine
Glutamine
Mechanism Problems
Question 25.30
The same and not the same. Write out mechanisms for the conversion of phosphoribosylamine into glycinamide ribonucleotide and of xanthylate into guanylate.
Question 25.31
Closing the ring. Propose a mechanism for the conversion of 5-
Chapter Integration Problems
Question 25.32
Different strokes. Human beings contain two different carbamoyl phosphate synthetase enzymes. One uses glutamine as a substrate, whereas the other uses ammonia. What are the functions of these two enzymes?
Question 25.33
A generous donor. What major biosynthetic reactions utilize PRPP?
Question 25.34
They’re everywhere! Nucleotides play a variety of roles in the cell. Give an example of a nucleotide that acts in each of the following roles or processes.
Second messenger
Phosphoryl-
group transfer Activation of carbohydrates
Activation of acetyl groups
Transfer of electrons
DNA sequencing
Chemotherapy
Allosteric effector
Question 25.35
Pernicious anemia. Purine biosynthesis is impaired by vitamin B12 deficiency. Why? How might fatty acid and amino acid metabolism also be affected by a vitamin B12 deficiency?
Question 25.36
Folate deficiency. Suppose someone was suffering from a folate deficiency. What cells would you think might be most affected? Symptoms may include diarrhea and anemia.
Question 25.37
Hyperuricemia. Many patients with glucose 6-
Question 25.38
Labeled carbon. Succinate uniformly labeled with 14C is added to cells actively engaged in pyrimidine biosynthesis. Propose a mechanism by which carbon atoms from succinate could be incorporated into a pyrimidine. At what positions is the pyrimidine labeled?
Question 25.39
Something funny going on here. Cells were incubated with glucose labeled with 14C in carbon 2, shown in red in the structure below. Later, uracil was isolated and found to contain 14C in carbons 4 and 6. Account for this labeling pattern.
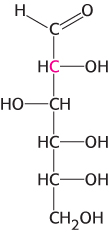
Question 25.40
Side effects. Azathioprine is an immunosuppressant drug used in kidney transplants. In vivo, azathioprine is metabolized to the hypoxanthine analog 6-
766
Question 25.41
Exercising muscle. Some interesting reactions take place in muscle tissue to facilitate the generation of ATP for contraction. In muscle contraction, ATP is converted into ADP. Adenylate kinase converts two molecules of ADP into a molecule of ATP and AMP.
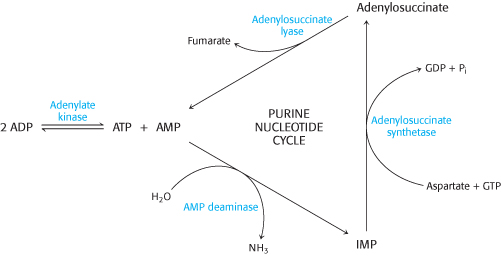
Why is this reaction beneficial to contracting muscle?
Why is the equilibrium for the adenylate kinase approximately equal to 1?
Muscle can metabolize AMP by using the purine nucleotide cycle. The initial step in this cycle, catalyzed by AMP deaminase, is the conversion of AMP into IMP.
Why might the deamination of AMP facilitate ATP formation in muscle?
How does the purine nucleotide cycle assist the aerobic generation of ATP?
Question 25.42
A common step. What three reactions transfer an amino group from aspartate to yield the aminated product and fumarate?
Question 25.43
Your pet duck. You suspect that your pet duck has gout. Why should you think twice before administering a dose of allopurinol-
Data Interpretation Problem
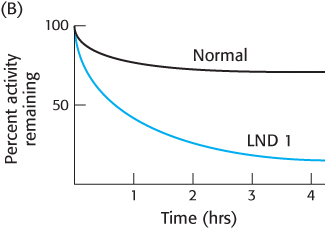
Question 25.44
Not all absences are equal. In order to better understand the nature of the defects in HGPRT in patients suffering from Lesch–
Describe the results shown in the figure.
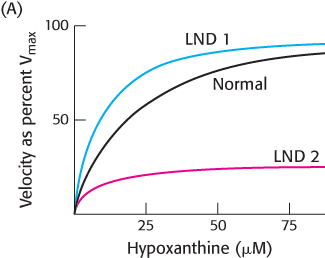
Suggest some possible explanations for the appearance of Lesch–
Nyhan disease even in the presence of apparently normal enzyme. The stability of the HGPRT was tested to determine if the LND 1 enzyme was more labile. The same amounts of the normal and LND 1 enzyme were incubated at 37 °C in the absence of any substrates or products. At various times, a portion of the enzyme was removed and assayed for enzyme activity. The results are shown in Figure B. Explain these results.