33.1A Wide Variety of Organic Compounds Are Detected by Olfaction
A Wide Variety of Organic Compounds Are Detected by Olfaction
Human beings can detect and distinguish thousands of different compounds by smell, often with considerable sensitivity and specificity. Most odorants are small organic compounds with sufficient volatility that they can be carried as vapors into the nose. For example, a major component responsible for the odor of almonds is the simple aromatic compound benzaldehyde, whereas the sulfhydryl compound 3-
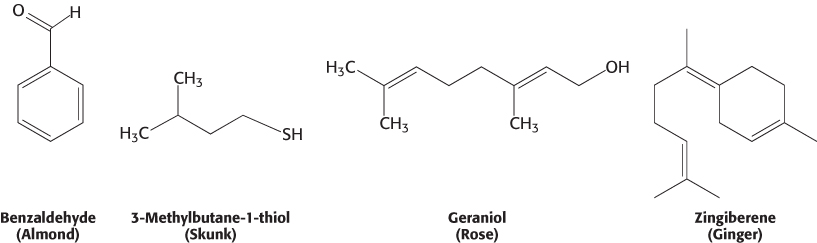
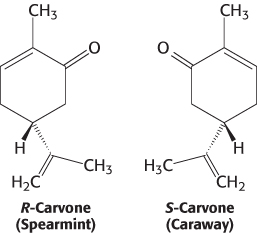
What properties of these molecules are responsible for their odors? First, the shape of the molecule rather than its other physical properties is crucial. We can most clearly see the importance of shape by comparing molecules such as those responsible for the odors of spearmint and caraway.
These compounds are identical in essentially all physical properties such as hydrophobicity because they are exact mirror images of one another. Thus, the odor produced by an odorant depends not on a physical property but on the compound’s interaction with a specific binding surface, most likely a protein receptor. Second, some human beings (and other animals) suffer from specific anosmias; that is, they are incapable of smelling specific compounds even though their olfactory systems are otherwise normal. Such anosmias are often inherited. These observations suggest that mutations in individual receptor genes lead to the loss of the ability to detect a small subset of compounds.
Olfaction is mediated by an enormous family of seven-transmembrane-helix receptors
Odorants are detected in a specific region of the nose, called the main olfactory epithelium, that lies at the top of the nasal cavity (Figure 33.2). Approximately 1 million sensory neurons line the surface of this region. Cilia containing the odorant-
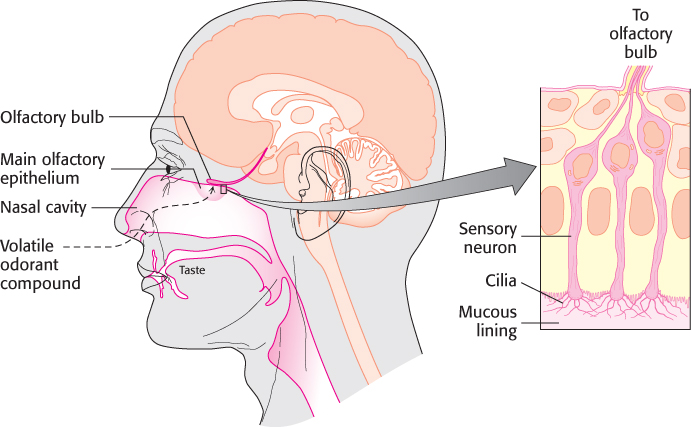
963
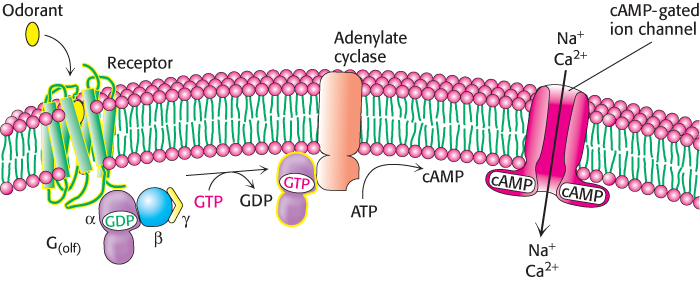
Biochemical studies in the late 1980s examined isolated cilia from rat olfactory epithelia that had been treated with odorants. Exposure to the odorants increased the cellular level of cyclic AMP, and this increase was observed only in the presence of GTP. On the basis of what was known about signal-
 The odorant receptor (hereafter, OR) family is even larger than expected: more than 1000 OR genes are present in the mouse and the rat, whereas the human genome encodes approximately 350 ORs. In addition, the human genome includes approximately 500 OR pseudogenes containing mutations that prevent the generation of a full-
The odorant receptor (hereafter, OR) family is even larger than expected: more than 1000 OR genes are present in the mouse and the rat, whereas the human genome encodes approximately 350 ORs. In addition, the human genome includes approximately 500 OR pseudogenes containing mutations that prevent the generation of a full-
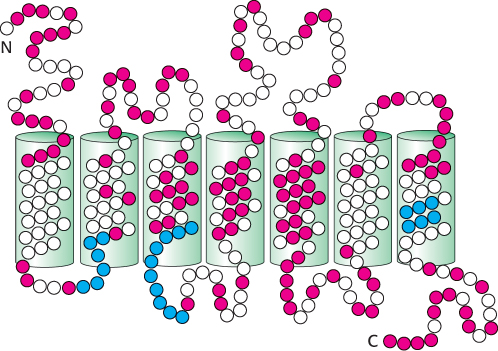
The OR proteins are typically 20% identical in sequence with the β-adrenergic receptor (Section 14.1) and from 30% to 60% identical with one another. Several specific sequence features are present in most or all OR family members (Figure 33.4). The central region, particularly transmembrane helices 4 and 5, is highly variable, suggesting that this region is the site of odorant binding. That site must be different in odorant receptors that bind distinct odorant molecules.
964
What is the relation between OR gene expression and the individual neuron? Interestingly, each olfactory neuron expresses only a single OR gene, among hundreds available. Apparently, the precise OR gene expressed is determined largely at random. After one OR gene is expressed and a functional OR protein is produced, the expression of all other OR genes is suppressed by a feedback mechanism that remains to be fully elucidated.
The binding of an odorant to an OR on the neuronal surface initiates a signal-
Odorants are decoded by a combinatorial mechanism
An obvious challenge presented to an investigator by the large size of the OR family is to match each OR with the one or more odorant molecules to which it binds. Exciting progress has been made in this regard. Initially, an OR was matched with odorants by overexpressing a single, specific OR gene in rats. This OR responded to straight-
965
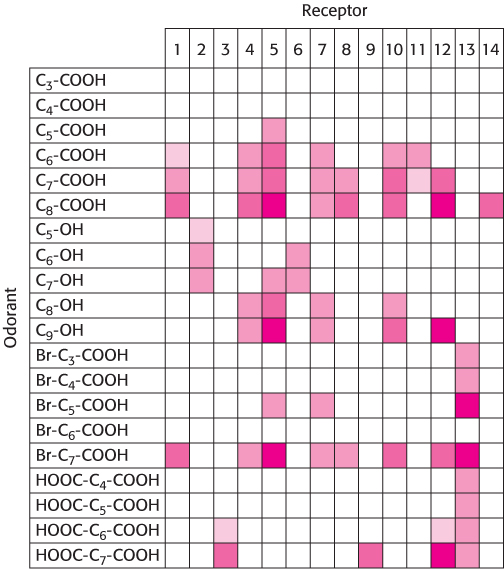
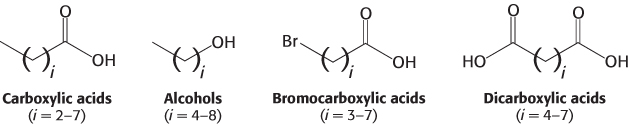
Using this approach, investigators analyzed the responses of neurons to a series of compounds having varying chain lengths and terminal functional groups (Figure 33.6). The results of these experiments appear surprising at first glance (Figure 33.7). Importantly, there is not a simple 1:1 correspondence between odorants and receptors. Almost every odorant activates a number of receptors (usually to different extents) and almost every receptor is activated by more than one odorant. Note, however, that each odorant activates a unique combination of receptors. In principle, this combinatorial mechanism allows even a small array of receptors to distinguish a vast number of odorants.
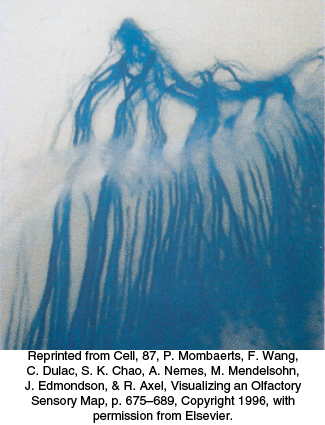
How is the information about which receptors have been activated transmitted to the brain? Recall that each neuron expresses only one OR and that the pattern of expression appears to be largely random. A substantial clue to the connections between receptors and the brain has been provided by the creation of mice that express a gene for an easily detectable colored marker in conjunction with a specific OR gene. Olfactory neurons that express the OR–
Can such a combinatorial mechanism truly distinguish many different odorants? An electronic “nose” that functions by the same principles provides compelling evidence that it can (Figure 33.9). The receptors for the electronic nose are polymers that bind a range of small molecules. Each polymer binds every odorant, but to varying degrees. Importantly, the electrical properties of these polymers change on odorant binding. A set of 32 of these polymer sensors, wired together so that the pattern of responses can be evaluated, is capable of distinguishing individual compounds such as n-pentane and n-hexane as well as complex mixtures such as the odors of fresh and spoiled fruit.

966