PROBLEMS
PROBLEMS
Question 34.1
First things first. Distinguish between the innate and adaptive immune systems.
Question 34.2
Antibody diversity. What are the mechanisms used by B cells to generate antibody diversity?
Question 34.3
Hang in there. Explain the difference between affinity and avidity. For which immunoglobulin class might avidity be particularly important in antigen recognition?
Question 34.4
Innate abilities. A strain of mice has been identified that does not respond to LPS. This lack of response is due to a single amino acid change in the intracellular domain of mouse TLR4. Propose an explanation for the lack of response.
Question 34.5
TLR ligands. The PAMP recognized by TLR3 is double-
Question 34.6
Energetics and kinetics. Suppose that the dissociation constant of an Fab–hapten complex is 3 × 10−7 M at 25°C.
What is the standard free energy of binding?
Immunologists often speak of affinity (Ka), the reciprocal of the dissociation constant, in comparing antibodies. What is the affinity of this Fab?
The rate constant for the release of hapten from the complex is 120 s−1. What is the rate constant for association? What does the magnitude of this value imply about the extent of structural change in the antibody on binding hapten?
Question 34.7
A brilliant emitter. Certain naphthalene derivatives, such as the dansyl group, exhibit a weak yellow fluorescence when they are in a highly polar environment (such as water) and an intense blue fluorescence when they are in a markedly nonpolar environment (such as hexane). The binding of ε-dansyl-
Question 34.8
Miniantibody. The Fab fragment of an antibody molecule has essentially the same affinity for a monovalent hapten as does intact IgG.
What is the smallest unit of an antibody that can retain the specificity and binding affinity of the whole protein?
Design a compact single-
chain protein that is likely to specifically bind antigen with high affinity.
Question 34.9
Turning on B cells. B lymphocytes, the precursors of plasma cells, are triggered to proliferate by the binding of multivalent antigens to receptors on their surfaces. The cell-
What do these findings reveal about the mechanism of B-
cell activation? How might antibodies be used to activate B cells?
Question 34.10
An ingenious cloning strategy. In the cloning of the gene for the α chain of the T-
1010
Question 34.11
Pathogen susceptibility. Patients carrying specific mutations in the gene encoding the TLR4 protein are susceptible to infections from Gram-
Question 34.12
Matchmaker, matchmaker. Why is it important to match HLA alleles between donor and recipient in organ transplantation?
Question 34.13
Instruction. Before the mechanism for generating antibody diversity had been established, a mechanism based on protein folding around an antigen was proposed, primarily by Linus Pauling. In this model, antibodies that had different specificities had the same amino acid sequence but were folded in different ways. Propose a test of this model.
Question 34.14
Dealing with nonsense. Cells, including immune cells, degrade mRNA molecules in which no long open reading frame is present. The process is called nonsense-
Question 34.15
Down, but not out. To understand the genes responsible for growth and infectivity in a disease-
Question 34.16
Presentation. The amino acid sequence of a small protein is
MSRLASKNLIRSDHAGGLLQATYSAVSS-
Predict the most likely peptide to be presented by the class I MHC molecule HLA-
Mechanism Problem
Question 34.17
Catalytic antibody. Antibody is generated against a transition state for the hydrolysis of the following ester.
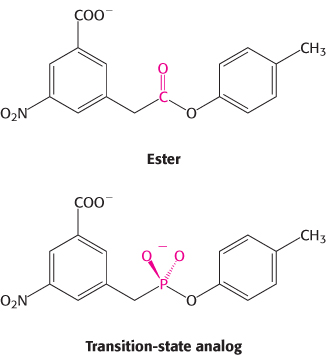
Some of these antibodies catalyze the hydrolysis of the ester. What amino acid residue might you expect to find in the binding site on the antibody?
Chapter Integration Problem
Question 34.18
Signaling. Protein tyrosine phosphatases, such as the molecule CD45 expressed in both B cells and T cells, play important roles in activating such protein tyrosine kinases as Fyn and Lck, which are quite similar to Src. Suggest a mechanism for the activation of such protein kinases by the removal of a phosphoryl group from a phosphotyrosine residue.
Data Interpretation Problem
Question 34.19
Affinity maturation. A mouse is immunized with an oligomeric human protein. Shortly after immunization, a cell line that expresses a single type of antibody molecule (antibody A) is derived. The ability of antibody A to bind the human protein is assayed, with the results shown in the graph below.
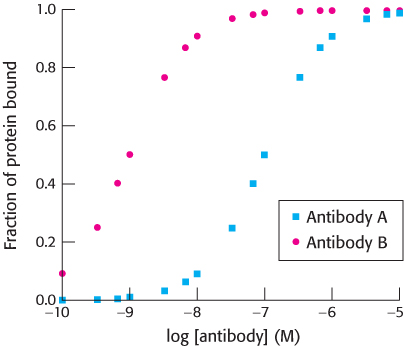
After repeated immunizations with the same protein, another cell line is derived that expresses a different antibody (antibody B). The results of analyzing the binding of antibody B to the protein also are shown. From these data, estimate
the dissociation constant (Kd) for the complex between the protein and antibody A.
the dissociation constant for the complex between the protein and antibody B.
Comparison of the amino acid sequences of antibody A and antibody B reveals them to be identical except for a single amino acid. What does this finding suggest about the mechanism by which the gene encoding antibody B was generated?