PROBLEMS
PROBLEMS
Question 35.1
Diverse motors. Skeletal muscle, eukaryotic cilia, and bacterial flagella use different strategies for the conversion of free energy into coherent motion. Compare and contrast these motility systems with respect to (a) the free-
Question 35.2
You call that slow? At maximum speed, a kinesin molecule moves at a rate of 6400 Å per second. Given the dimensions of the motor region of a kinesin dimer of approximately 80 Å, calculate its speed in “body lengths” per second. To what speed does this body-
Question 35.3
Heavy lifting. A single myosin motor domain can generate a force of approximately 4 piconewtons (4 pN). How many times its “body weight” can a myosin motor domain lift? Note that 1 newton = 0.22 pounds (100 g). Assume a molecular mass of 100 kDa for the motor domain.
Question 35.4
Compare and contrast. Describe two similarities and two differences between actin filaments and microtubules.
Question 35.5
Lighten up. What is the primary role of the light chains in myosin? In kinesin?
Question 35.6
Rigor mortis. Propose an explanation for the fact that the body stiffens after death.
Question 35.7
Now you see it, now you don’t. Under certain stable concentration conditions, actin monomers in their ATP form will polymerize to form filaments that disperse again into free actin monomers over time. Explain.
Question 35.8
Helicases as motors. Helicases can use single-
Question 35.9
New moves. When bacteria such as E. coli are starved to a sufficient extent, they become nonmotile. However, when such bacteria are placed in an acidic solution, they resume swimming. Explain.
Question 35.10
Going straight. Suppose you measure the mean distance that an E. coli bacterium moves along a straight path before it tumbles over a period of time. Would you expect this distance to change in the presence of a gradient of a chemoattractant? Would it be longer or shorter?
Question 35.11
Hauling a load. Consider the action of a single kinesin molecule in moving a vesicle along a microtubule track. The force required to drag a spherical particle of radius a at a velocity v in a medium having a viscosity η is
F = 6πηav
Suppose that a 2-
What is the magnitude of the force exerted by the kinesin molecule? Express the value in dynes (1 dyne = 1 g cm s−2).
How much work is performed in 1 s? Express the value in ergs (1 erg = 1 dyne cm).
A kinesin motor hydrolyzes approximately 80 molecules of ATP per second. What is the energy associated with the hydrolysis of this much ATP in ergs? Compare this value with the actual work performed.
Question 35.12
Unusual strides. A publication describes a kinesin molecule that is claimed to move along microtubules with a step size of 6 nm. You are skeptical. Why?
Question 35.13
The sound of one hand clapping. KIF1A is a motor protein that moves toward the plus end of microtubules as a monomer. KIF1A has only a single motor domain. What additional structural elements would you expect to find in the KIF1A structure?
Question 35.14
Building blocks. Actin filaments, microtubules, and bacterial flagella are all built from small subunits. Describe three advantages of assembling long filamentous structures from subunits rather than from single, long proteins.
Mechanism Problem
Question 35.15
Backward rotation. On the basis of the proposed structure in Figure 35.29 for the bacterial flagellar motor, suggest a pathway for transmembrane proton flow when the flagellar motor is rotating clockwise rather than counterclockwise.
1032
Chapter Integration Problem
Question 35.16
Smooth muscle. Smooth muscle, in contrast with skeletal muscle, is not regulated by a tropomyosin–
Data Interpretation Problem
Question 35.17
Myosin V. An abundant myosin-
The rate of ATP hydrolysis by myosin has been examined as a function of ATP concentration, as shown in graph A.
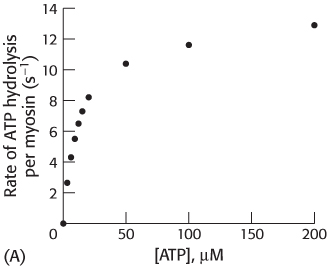
Estimate the values of kcat and KM for ATP.
With the use of optical-
trap measurements, the motion of single myosin V dimers could be followed, as shown in graph B. 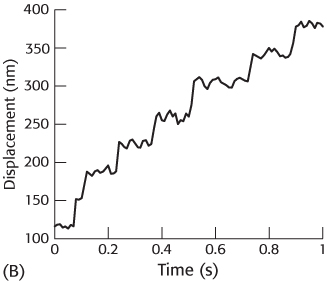 [Based on M. Rief et al., Proc. Natl. Acad. Sci. U. S. A. 97:9482–
[Based on M. Rief et al., Proc. Natl. Acad. Sci. U. S. A. 97:9482–9486, 2000.] Estimate the step size for myosin V.
The rate of ADP release from myosin V is found to be approximately 13 molecules per second.
Combine the observations about the amino acid sequence of myosin, the observed step size, and the kinetics results to propose a mechanism for the processive motion of myosin V.