9.2Carbonic Anhydrases Make a Fast Reaction Faster
Carbonic Anhydrases Make a Fast Reaction Faster
Carbon dioxide is a major end product of aerobic metabolism. In mammals, this carbon dioxide is released into the blood and transported to the lungs for exhalation. While in the red blood cells, carbon dioxide reacts with water (Section 7.3). The product of this reaction is a moderately strong acid, carbonic acid (pKa = 3.5), which is converted into bicarbonate ion 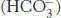 on the loss of a proton.
on the loss of a proton.
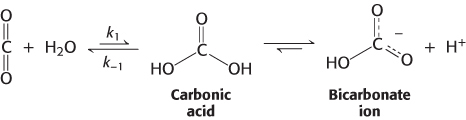
Even in the absence of a catalyst, this hydration reaction proceeds at a moderately fast pace. At 37°C near neutral pH, the second- , is even more rapid, with a rate constant of k−1 = 50 s−1. These rate constants correspond to an equilibrium constant of K1 = 5.4 × 10−5 and a ratio of [CO2] to [H2CO3] of 340 : 1 at equilibrium.
, is even more rapid, with a rate constant of k−1 = 50 s−1. These rate constants correspond to an equilibrium constant of K1 = 5.4 × 10−5 and a ratio of [CO2] to [H2CO3] of 340 : 1 at equilibrium.
 Carbon dioxide hydration and
Carbon dioxide hydration and 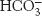 dehydration are often coupled to rapid processes, particularly transport processes. Thus, almost all organisms contain enzymes, referred to as carbonic anhydrases, that increase the rate of reaction beyond the already reasonable spontaneous rate. For example, carbonic anhydrases dehydrate
dehydration are often coupled to rapid processes, particularly transport processes. Thus, almost all organisms contain enzymes, referred to as carbonic anhydrases, that increase the rate of reaction beyond the already reasonable spontaneous rate. For example, carbonic anhydrases dehydrate 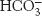 in the blood to form CO2 for exhalation as the blood passes through the lungs. Conversely, they convert CO2 into
in the blood to form CO2 for exhalation as the blood passes through the lungs. Conversely, they convert CO2 into 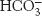 to generate the aqueous humor of the eye and other secretions. Furthermore, both CO2 and
to generate the aqueous humor of the eye and other secretions. Furthermore, both CO2 and 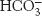 are substrates and products for a variety of enzymes, and the rapid interconversion of these species may be necessary to ensure appropriate substrate levels. So important are these enzymes in human beings that mutations in some carbonic anhydrases have been found to be associated with osteopetrosis (excessive formation of dense bones accompanied by anemia) and mental retardation.
are substrates and products for a variety of enzymes, and the rapid interconversion of these species may be necessary to ensure appropriate substrate levels. So important are these enzymes in human beings that mutations in some carbonic anhydrases have been found to be associated with osteopetrosis (excessive formation of dense bones accompanied by anemia) and mental retardation.
Carbonic anhydrases accelerate CO2 hydration dramatically. The most-
265
Carbonic anhydrase contains a bound zinc ion essential for catalytic activity
Less than 10 years after the discovery of carbonic anhydrase in 1932, this enzyme was found to contain a bound zinc ion. Moreover, the zinc ion appeared to be necessary for catalytic activity. This discovery, remarkable at the time, made carbonic anhydrase the first known zinc-
X-
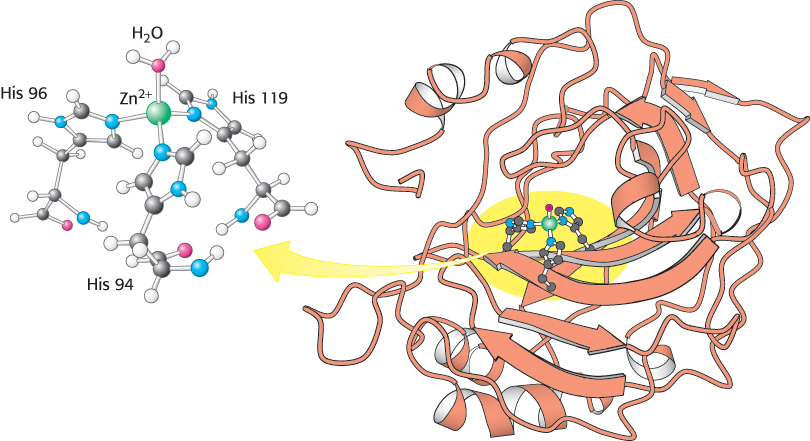
 FIGURE 9.21 The structure of human carbonic anhydrase II and its zinc site. (Left) Notice that the zinc ion is bound to the imidazole rings of three histidine residues as well as to a water molecule. (Right) Notice the location of the zinc site in a cleft near the center of the enzyme.
FIGURE 9.21 The structure of human carbonic anhydrase II and its zinc site. (Left) Notice that the zinc ion is bound to the imidazole rings of three histidine residues as well as to a water molecule. (Right) Notice the location of the zinc site in a cleft near the center of the enzyme.
Zinc is found only in the +2 state in biological systems. A zinc atom is essentially always bound to four or more ligands; in carbonic anhydrase, three coordination sites are occupied by the imidazole rings of three histidine residues and an additional coordination site is occupied by a water molecule (or hydroxide ion, depending on pH). Because the molecules occupying the coordination sites are neutral, the overall charge on the Zn(His)3 unit remains +2.
Catalysis entails zinc activation of a water molecule
How does this zinc complex facilitate carbon dioxide hydration? A major clue comes from the pH profile of enzymatically catalyzed carbon dioxide hydration (Figure 9.22).
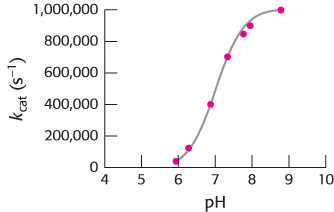
At pH 8, the reaction proceeds near its maximal rate. As the pH decreases, the rate of the reaction drops. The midpoint of this transition is near pH 7, suggesting that a group that loses a proton at pH 7 (pKa = 7) plays an important role in the activity of carbonic anhydrase. Moreover, the curve suggests that the deprotonated (high pH) form of this group participates more effectively in catalysis. Although some amino acids, notably histidine, have pKa values near 7, a variety of evidence suggests that the group responsible for this transition is not an amino acid but is the zinc-
266
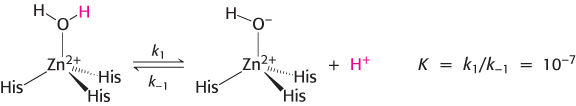
The binding of a water molecule to the positively charged zinc center reduces the pKa of the water molecule from 15.7 to 7 (Figure 9.23). With the pKa lowered, the water molecule can more easily lose a proton at neutral pH, generating a substantial concentration of hydroxide ion (bound to the zinc atom). A zinc-
The zinc ion facilitates the release of a proton from a water molecule, which generates a hydroxide ion.
The carbon dioxide substrate binds to the enzyme’s active site and is positioned to react with the hydroxide ion.
The hydroxide ion attacks the carbon dioxide, converting it into bicarbonate ion,
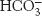 .
.The catalytic site is regenerated with the release of
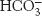 and the binding of another molecule of water.
and the binding of another molecule of water.
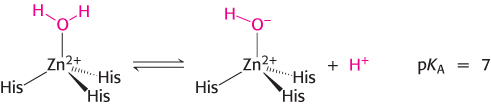
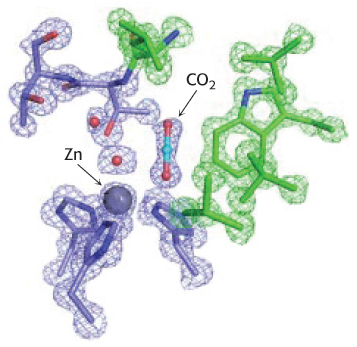
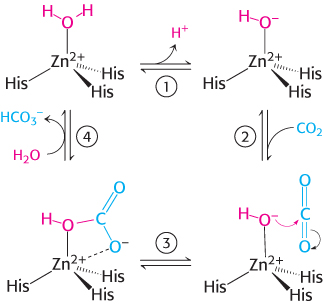
Thus, the binding of a water molecule to the zinc ion favors the formation of the transition state by facilitating proton release and by positioning the water molecule to be in close proximity to the other reactant.
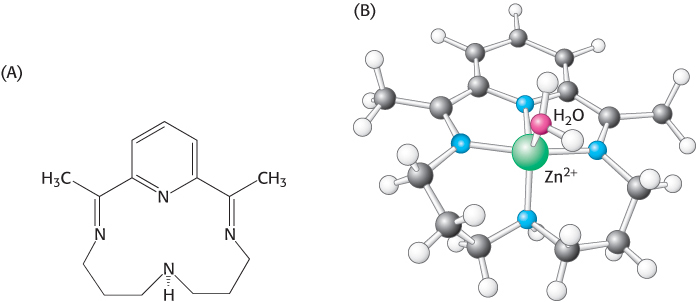
Studies of a synthetic analog model system provide evidence for the mechanism’s plausibility. A simple synthetic ligand binds zinc through four nitrogen atoms (compared with three histidine nitrogen atoms in the enzyme), as shown in Figure 9.26. One water molecule remains bound to the zinc ion in the complex. Direct measurements reveal that this water molecule has a pKa value of 8.7, not as low as the value for the water molecule in carbonic anhydrase but substantially lower than the value for free water. At pH 9.2, this complex accelerates the hydration of carbon dioxide more than 100-
267
A proton shuttle facilitates rapid regeneration of the active form of the enzyme
As noted earlier, some carbonic anhydrases can hydrate carbon dioxide at rates as high as a million times a second (106 s−1). The magnitude of this rate can be understood from the following observations. In the first step of a carbon dioxide hydration reaction, the zinc-
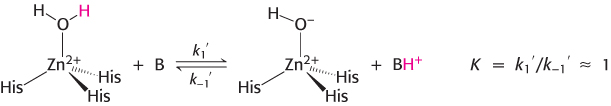
The answer became clear with the realization that the highest rates of carbon dioxide hydration require the presence of buffer, suggesting that the buffer components participate in the reaction. The buffer can bind or release protons. The advantage is that, whereas the concentrations of protons and hydroxide ions are limited to 10−7 M at neutral pH, the concentration of buffer components can be much higher, of the order of several millimolar. If the buffer component BH+ has a pKa of 7 (matching that for the zinc-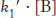 . The second-
. The second-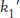 and
and 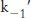 will be limited by buffer diffusion to values less than approximately 109 M−1 s−1. Thus, buffer concentrations greater than [B] = 10−3 M (or 1 mM) may be high enough to support carbon dioxide hydration rates of 106 M−1 s−1 because
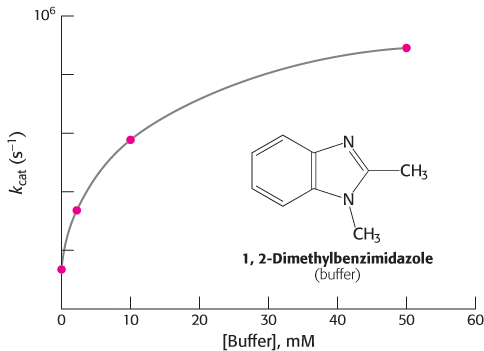
will be limited by buffer diffusion to values less than approximately 109 M−1 s−1. Thus, buffer concentrations greater than [B] = 10−3 M (or 1 mM) may be high enough to support carbon dioxide hydration rates of 106 M−1 s−1 because 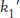 · [B] = (109 M−1s−1) · (10−3M) = 106 s−1. The prediction that the rate increases with increasing buffer concentration has been confirmed experimentally (Figure 9.29).
· [B] = (109 M−1s−1) · (10−3M) = 106 s−1. The prediction that the rate increases with increasing buffer concentration has been confirmed experimentally (Figure 9.29).
268
The molecular components of many buffers are too large to reach the active site of carbonic anhydrase. Carbonic anhydrase II has evolved a proton shuttle to allow buffer components to participate in the reaction from solution. The primary component of this shuttle is histidine 64. This residue transfers protons from the zinc-

269