 From Genotype to Phenotype
From Genotype to Phenotype
phenotype The observable characteristics of a person, including appearance, personality, intelligence, and all other traits.
As already explained, when a sperm and an ovum combine into a zygote, they establish the genotype: all the genes that the developing person has. Creation of a person from one cell involves several complex processes to form the phenotype—the person’s appearance, behavior, and brain and body functions. Nothing is totally genetic, not even such obvious traits as height or hair color, but nothing is untouched by genes, not even behavior such as voting Republican or Democrat, working overtime or not at all, wanting or refusing a divorce (Plomin et al., 2013).
polygenic Referring to a trait that is influenced by many genes.
multifactorial Referring to a trait that is affected by many factors, both genetic and environmental, that enhance, halt, shape, or alter the expression of genes, resulting in a phenotype that may differ markedly from the genotype.
The genotype instigates body and brain formation, but the phenotype depends on many genes and on the environment, influenced from the moment of conception until the moment of death through “the organism’s encounters with its prenatal and postnatal environments” (Gilbert, 2010, p. 26). Most traits are polygenic (affected by many genes) and multifactorial (influenced by many factors). A zygote might have the genes for becoming, say, a musical genius, but that potential may never be expressed. Completely accurate prediction of a person’s phenotype is impossible, even if the genotype is entirely known (Lehner, 2013).
Almost daily, researchers describe additional complexities in polygenic and multifactorial interaction. It is apparent that “phenotypic variation … results from multiple interactions among numerous genetic and environmental factors.” To describe this “fundamental problem of interrelating genotype and phenotype in complex traits” (Nadeau & Dudley, 2011, p. 1015), we begin with epigenetics.
Epigenetics
As you learned in Chapter 1, all important human characteristics are epigenetic, including diseases known to be inherited such as cancer, schizophrenia, and autism (Kundu, 2013; Plomin et al., 2013).
Diabetes is a notable example. People who inherit genes that put them at risk might nonetheless never develop diabetes. Alternatively, at some point, factors in the diet might activate that genetic risk, causing the person to become diabetic. Once that happens, epigenetic changes in the genes make diabetes irreversible: diet and insulin may control the disease, allowing the person to live a normal life, but the pre-diabetic state never returns (Reddy & Natarajan, 2013).
The same may be true for other developmental changes over the life span. Certain environmental influences (such as injury, temperature extremes, drug abuse, and crowding) can impede healthy development, whereas others (nourishing food, loving care, play) can facilitate it, all because of differential susceptibility. A recent discovery is that some epigenetic factors that suppress or release genes are cognitive, not biological. For example, if a person feels lonely and rejected, that feeling can affect the RNA (organic material that enables DNA to function), which allows genetic potential for heart disease or social anxiety to be expressed (Slavich & Cole, 2013).
No trait—even one with strong, proven, genetic origins, such as blood pressure or severe depression—is determined by genes alone because “development is an epigenetic process that entails cascades of interactions across multiple levels of causation, from genes to environments” (Spencer et al., 2009, p. 80). Because epigenetic influences occur lifelong, latent genes can become activated at any point.
A surprising example might be political ideology: Several researchers report that a particular allele of a dopamine receptor gene (DRD4-R7) correlates with being liberal, but only if a person has many friends. Loners, even with the liberal-leaning allele, are more conservative (Settle et al., 2010).
Especially for Future Parents Suppose you wanted your daughters to be short and your sons to be tall. Could you achieve that?
Response for Future Parents: Possibly, but you wouldn’t want to. You would have to choose one mate for your sons and another for your daughters, and you would have to use sex-selection methods. Even so, it might not work, given all the genes on your genotype. More important, the effort would be unethical, unnatural, and possibly illegal.

Gene-Gene Interactions
Human Genome Project An international effort to map the complete human genetic code. This effort was essentially completed in 2001, though analysis is ongoing.
Many discoveries have followed the completion of the Human Genome Project in 2001. One of the first surprises was that humans have far fewer than 100,000 genes, the number often cited in the twentieth century. The total number of genes in a person is between 18,000 and 23,000. The precise number is elusive because—another surprise—it is not always easy to figure out where one gene starts and another ends, or even if a particular stretch of DNA is actually a gene (Rouchka & Cha, 2009). Nor is it always easy to predict exactly how the genes from one parent will interact with the genes from the other. We do, however, know some basics of genetic interaction, which we describe now.
Additive Heredity
Some alleles are additive because their effects add up to influence the phenotype. When genes interact additively, the phenotype usually reflects the contributions of every gene that is involved. Height, hair curliness, and skin color, for instance, are usually the result of additive genes. Indeed, height is probably influenced by 180 genes, each contributing a very small amount (Enserink, 2011).
Most people have ancestors of varied height, hair curliness, skin color, and so on, so their children’s phenotype does not mirror the parents’ phenotypes (although the phenotype does always reflect their genotypes). I see this in my family: Our daughter Rachel is of average height, shorter than her father or me, but taller than either of our mothers. She apparently inherited some of her grandmothers’ height genes from us. And none of my children have exactly my skin color—apparent when we borrow clothes from each other and are distressed that a particular shade is attractive on one but ugly on another.
How any additive trait turns out depends partly on all the genes a child happens to inherit (half from each parent, which means one-fourth from each grandparent). Some genes amplify or dampen the effects of other genes, aided by all the other DNA and RNA (not junk!) in the zygote (Pauler et al., 2012).
Dominant-Recessive Heredity
dominant-recessive pattern The interaction of a heterozygous pair of alleles in such a way that the phenotype reflects one allele (the dominant gene) more than the other (the recessive gene).
carrier A person whose genotype includes a gene that is not expressed in the phenotype. The carried gene occurs in half of the carrier’s gametes and thus is passed on to half of the carrier’s children. If such a gene is inherited from both parents, the characteristic appears in the phenotype.
Not all genes are additive. In one nonadditive form, alleles interact in a dominant–
Most recessive genes are harmless. For example, blue eyes are determined by a recessive allele and brown eyes by a dominant one, so a child conceived by a blue-eyed parent (who always has two recessive blue-eye genes) and a brown-eyed parent will usually have brown eyes.
“Usually have brown eyes” is accurate because sometimes a brown-eyed person is a carrier of the blue-eye gene. In that case, in a blue-eye/brown-eye couple, every child has at least one blue-eye gene (from the blue-eyed parent) and half of them will have a blue-eye recessive gene (from the brown-eyed parent). Then half of those will have blue eyes because they have no dominant brown-eye gene. The other half will have a brown-eye dominant gene and thus have brown eyes but be carriers, like their brown-eyed parent.
This gets tricky if both parents are carriers. Thus if two brown-eyed parents both have the blue-eye recessive gene, they could have a blue-eyed child (one chance in four). No one should raise doubts about the child’s paternity (see Figure 3.4). The same pattern is found for all recessive genes: Most are harmless (like the genes for blue eyes) but some are lethal (like those that cause serious diseases, discussed at the end of this chapter.)
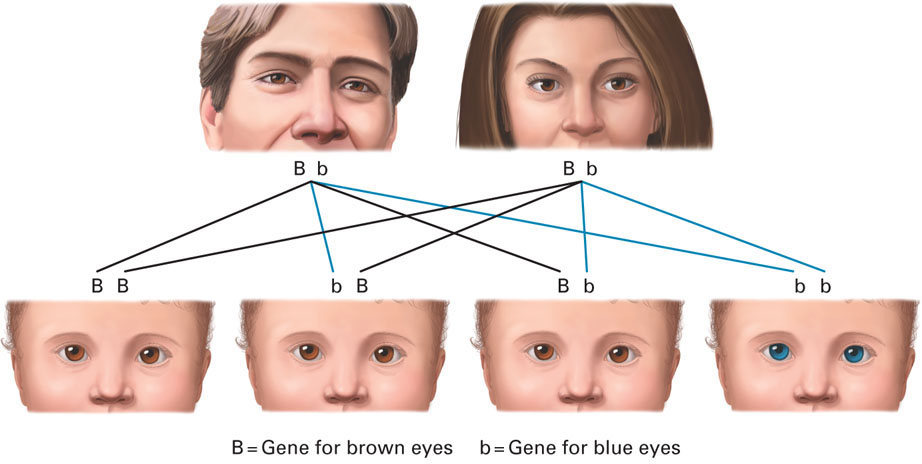
FIGURE 3.4
Changeling? No. If two brown-eyed parents both carry the blue-eye gene, they have one chance in four of having a blue-eyed child. Other recessive genes include the genes for red hair, Rh negative blood, and many genetic diseases.X-linked A gene carried on the X chromosome. If a male inherits an X-linked recessive trait from his mother, he expresses that trait because the Y from his father has no counteracting gene. Females are more likely to be carriers of X-linked traits but are less likely to express them.
A special case of the dominant–
| 23rd Pair | Phenotype | Genotype | Next Generation |
|---|---|---|---|
| 1. XX | Normal woman | Not a carrier | No color blindness from mother |
| 2. XY | Normal man | Normal X from mother | No color blindness from father |
| 3. XX | Normal woman | Carrier from father | Half her children will inherit her X. The girls with her X will be carriers; the boys with her X will be color-blind. |
| 4. XX | Normal woman | Carrier from mother | Half her children will inherit her X. The girls with her X will be carriers; the boys with her X will be color-blind. |
| 5. XY | Color-blind man | Inherited from mother | All his daughters will have his X. None of his sons will have his X. All his children will have normal vision, unless their mother also had an X for color blindness. |
| 6. XX | Color-blind woman (rare) | Inherited from both parents | Every child will have one X from her. Therefore, every son will be color-blind. Daughters will be only carriers, unless they also inherit an X from the father, as their mother did. |
To understand the inheritance of X-linked traits, you need to remember that the Y chromosome is much smaller than the X, so genes on the X almost never have a counterpart on the Y. Therefore, recessive traits carried on the X affect the phenotypes of sons more often than daughters because the daughters have another X chromosome, usually with the dominant gene. This explains why males with an X-linked disorder inherited it from their mothers, not their fathers. Because of their mothers, 20 times more boys than girls are color-blind (McIntyre, 2002).
Copy Number Variations
For any living creature, the outcomes of all the interactions involved in heredity are difficult to predict. A small deletion, repetition, or transposition in any of the 3 billion base pairs may be inconsequential, lethal, or something in between.
When the human genome was first mapped in 2001, it was hoped that a specific additive, recessive, or dominant gene could be located for each genetic disorder and that a cure would soon follow. That “one gene/one disorder” hope proved to be fantasy, disappointing many doctors who hoped that personalized medicine was imminent (Marshall, 2011). Molecular analysis found, instead, that thousands of seemingly minor variations in base pairs turn out to be influential—each in small ways. Since there are 3 billion combinations of base pairs, accumulated variations have notable impact.
copy number variations Genes with various repeats or deletions of base pairs.
Attention has focused on copy number variations, which are genes with repeats (from one to hundreds) or deletions of base pairs. Copy number variations correlate with almost every disease and condition, including heart disease, impaired intellectual abilities, mental illness, and many cancers. Such variations are partly developmental in that they are particularly influential prenatally as basic brain structures are formed. But remember plasticity—changes can occur lifelong (Chaignat et al., 2011).
Copy number variations are “abundant”—we all have some of them (Mills et al., 2011). Detecting and interpreting such variations may be crucial for personalized medicine in the future. For instance, many drugs work differently depending on the genetic structure of the recipient. These specific factors lead directly to effective treatment of individual patients (Marshall, 2011).
Researchers are just beginning to understand the implications of copy number variations, although many hope that soon such genetic information will help target drugs and other medical measures. Even that may be a hopeful fantasy. Since epigenetics shows that environmental influences can actually change genetic expression, personalized medicine must consider each individual’s habits of mind and life at least as much as their genes (Horowitz et al., 2013)
To further complicate matters, sometimes one-half of a gene pair switches off completely, allowing the other free rein but potentially causing a problem if that remaining gene has a deleterious variation. For girls, one X of the 23rd pair is deactivated early in prenatal life. The implications of that shut-off are not well understand, but it is known that sometimes that X is from the ovum, sometimes from the sperm. Boys, of course, have only one X, so it always is activated.
That is not the only case that is affected by sex. Sometimes the same gene affects male and female embryos differently. It also matters whether a gene came from the mother or the father, a phenomenon called parental imprinting.
The best-known example of parental imprinting occurs with a small deletion on chromosome 15. If that deletion came from the father’s chromosome 15, the child may develop Prader-Willi syndrome and be obese, slow moving, and stubborn. If that deletion came from the mother’s chromosome 15, the child will have Angel-man syndrome and be thin, hyperactive, and happy—sometimes too happy, laughing when no one else does. In both cases, intellectual development is impaired, though in somewhat distinct ways.
Parental imprinting is quite common. Early in prenatal development (day 15), an estimated 553 genes act differently if they come from the mother or from the father—a much higher frequency than previously thought (Gregg, 2010). Imprinting may be affected by the sex of the embryo as well as the sex of the parent. For instance, women develop multiple sclerosis more often than men, and they usually inherit it from their mothers, not their fathers, probably for genetic as well as epigenetic reasons (Huynh & Casaccia, 2013).

SUMMING UP
The distinction between genotype (heredity) and phenotype (manifest appearance and observed behavior) is one of the many complexities in genetics and development. All traits are epigenetic, the product of genetic and nongenetic influences, beginning with methylation at conception and continuing lifelong. Furthermore, most traits are polygenic, the result of many genes that interact—some additively and some in a dominant–