An atom of hydrogen has one electron in its first shell. One more electron is needed to fill this shell. In a molecule of H2, hydrogen atoms form a strong bond by sharing two electrons.

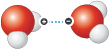
A weak bond is formed between the slightly positively charged hydrogen atom of one water molecule (H2O) and the slightly negatively charged oxygen atom of another.
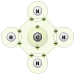
In methane (CH4), four hydrogen atoms and one carbon atom share electrons to complete the outermost shells of each atom.
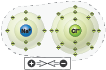
A chlorine atom can “take” the lone electron in sodium’s outermost shell to complete its own outermost shell. As a result, both atoms strongly interact with each other through opposite charges forming sodium chloride (NaCl), a salt.
Two pairs of electrons are shared between two oxygen atoms in a molecule of oxygen gas (O2), forming a strong bond.

covalent
ionic
hydrogen