
The bonds between water molecules are closer together when water is in liquid form compared to when it is frozen solid. Therefore, water expands when it freezes. This allows ice to float on top of liquid water.
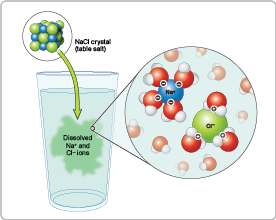
Ionic compounds like sodium chloride (NaCl)—better known as table salt—are readily broken down into sodium ions (Na+) and chloride ions (Cl-). In turn, positively charged Na+ ions are attracted to the negatively charged side of H2O molecules, and negatively charged Cl- ions are attracted to the positively charged side of H2O molecules.

Water molecules stick together with substantial strength as the result of bonds between water molecules. The bonds are strong enough to allow some animals, such as the fishing spider and water strider, the ability to walk on water.

The “sticky” bonds between H2O molecules allow water to be pulled through a tree’s root system, all the way up to its leaves. Trees such as the giant sequoia are capable of transporting water molecules from the soil to leaves 300 ft. above the ground!

Rather than raising the temperature of water, heat from the sun mostly disrupts and reorganizes the bonds between water molecules. This explains why, on a sunny day at the beach, the sand may be extremely hot while the water is many degrees cooler.